Association of sirolimus adverse effects with m-TOR, p70S6K or Raptor polymorphisms in kidney transplant recipients
- PMID: 22863900
- PMCID: PMC3894420
- DOI: 10.1097/FPC.0b013e328357359d
Association of sirolimus adverse effects with m-TOR, p70S6K or Raptor polymorphisms in kidney transplant recipients
Abstract
Background: The mammalian target of rapamycin (m-TOR) inhibitor sirolimus is an immunosuppressive drug used in kidney transplantation. m-TOR binds with Raptor and phosphorylates p70S6 kinase, a protein involved in numerous cell signalling pathways. We examined the association of candidate polymorphisms in m-TOR, Raptor and p70S6K, sirolimus dose and exposure, and other time-independent as well as time-dependent covariates, with sirolimus-induced adverse events in kidney transplant recipients.
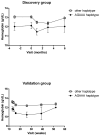
Methods: This study included a first group of 113 patients, switched from a calcineurin inhibitor to sirolimus, and a validation group of 66 patients from another clinical trial, with the same immunosuppressive regimen. The effects of gene polymorphisms and covariates on the total cholesterol, LDL cholesterol, triglycerides, haemoglobin, cutaneous adverse events, oedemas and infections were studied using multilinear regression, or logistic regression imbedded in linear mixed-effect models.
Results: An m-TOR variant haplotype was significantly associated with a decrease in haemoglobin levels in the two populations of patients (discovery group: β=-0.82 g/dl, P=0.0076; validation group: β=-1.58 g/dl, P=0.0308). Increased sirolimus trough levels were significantly associated with increased total cholesterol levels (discovery group: β=0.02 g/l, P<0.0001; validation group: β=0.02 g/l, P=0.0002) and triglyceride levels (discovery group: β=0.02 g/l, P=0.0059; validation group: β=0.05 g/l, P=0.0370). Sirolimus trough levels were also associated with an increased risk for cutaneous adverse events [odds ratio=1.97, 95% confidence interval (1.32-1.94), P=0.0009] and oedemas [odds ratio=1.16, 95% confidence interval (1.03-1.30), P=0.01342] in the discovery group, but this was not confirmed in the validation group.
Conclusion: These results provide evidence of an association between an m-TOR haplotype and a decrease in haemoglobin in renal transplant recipients.
Figures
Comment in
-
Pharmacogenetics in solid organ transplantation: a transition from kinetics to dynamics.Pharmacogenomics. 2012 Nov;13(15):1679-83. doi: 10.2217/pgs.12.155. Pharmacogenomics. 2012. PMID: 23171332 No abstract available.
References
-
- MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71:271–280. - PubMed
-
- Augustine JJ, Knauss TC, Schulak JA, Bodziak KA, Siegel C, Hricik DE. Comparative effects of sirolimus and mycophenolate mofetil on erythropoiesis in kidney transplant patients. Am J Transplant. 2004;4:2001–2006. - PubMed
-
- Mahe E, Morelon E, Lechaton S, Sang KH, Mansouri R, Ducasse MF, et al. Cutaneous adverse events in renal transplant recipients receiving sirolimus-based therapy. Transplantation. 2005;79:476–482. - PubMed
-
- Kahan BD. Sirolimus-based immunosuppression: present state of the art. J Nephrol. 2004;17 (Suppl 8):S32–39. - PubMed
-
- Morelon E, Stern M, Kreis H. Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med. 2000;343:225–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous