Ring expansion of alkynyl cyclopropanes to highly substituted cyclobutenes via a N-sulfonyl-1,2,3-triazole intermediate
- PMID: 22864054
- PMCID: PMC3717254
- DOI: 10.1039/c2cc34609e
Ring expansion of alkynyl cyclopropanes to highly substituted cyclobutenes via a N-sulfonyl-1,2,3-triazole intermediate
Abstract
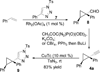
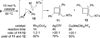
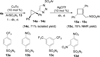
Regioselective ring expansion of alkynyl cyclopropanes to highly substituted cyclobutenes was developed. The reaction involves a copper-catalyzed cycloaddition of an alkyne with an arylsulfonyl azide and a silver-catalyzed carbene formation followed by ring expansion of a cyclopropyl carbene intermediate.
Figures
Similar articles
-
Enantioselective Cyclobutenylation of Olefins Using N-Sulfonyl-1,2,3-Triazoles as Vicinal Dicarbene Equivalents.Org Lett. 2021 Aug 20;23(16):6530-6535. doi: 10.1021/acs.orglett.1c02331. Epub 2021 Aug 10. Org Lett. 2021. PMID: 34374544
-
Alkyne-azide cycloaddition catalyzed by silver chloride and "abnormal" silver N-heterocyclic carbene complex.ScientificWorldJournal. 2013 Nov 6;2013:186537. doi: 10.1155/2013/186537. eCollection 2013. ScientificWorldJournal. 2013. PMID: 24307866 Free PMC article.
-
Theoretical studies on the regioselectivity of iridium-catalyzed 1,3-dipolar azide-alkyne cycloaddition reactions.J Org Chem. 2014 Dec 19;79(24):11970-80. doi: 10.1021/jo5018348. Epub 2014 Sep 25. J Org Chem. 2014. PMID: 25222638
-
Cu-catalyzed azide-alkyne cycloaddition.Chem Rev. 2008 Aug;108(8):2952-3015. doi: 10.1021/cr0783479. Chem Rev. 2008. PMID: 18698735 Review. No abstract available.
-
Computational studies on the regioselectivity of metal-catalyzed synthesis of 1,2,3 triazoles via click reaction: a review.J Mol Model. 2015 Oct;21(10):264. doi: 10.1007/s00894-015-2810-2. Epub 2015 Sep 18. J Mol Model. 2015. Retraction in: J Mol Model. 2019 Mar 7;25(4):86. doi: 10.1007/s00894-019-3978-7. PMID: 26385849 Retracted. Review.
Cited by
-
Generation of rhodium(I) carbenes from ynamides and their reactions with alkynes and alkenes.J Am Chem Soc. 2013 Jun 5;135(22):8201-4. doi: 10.1021/ja4047069. Epub 2013 May 23. J Am Chem Soc. 2013. PMID: 23701315 Free PMC article.
-
Stereoselective 1,3-insertions of rhodium(II) azavinyl carbenes.J Am Chem Soc. 2014 Jan 8;136(1):195-202. doi: 10.1021/ja408185c. Epub 2013 Dec 17. J Am Chem Soc. 2014. PMID: 24295389 Free PMC article.
-
Tandem 1,2-sulfur migration and (aza)-Diels-Alder reaction of β-thio-α-diazoimines: rhodium catalyzed synthesis of (fused)-polyhydropyridines, and cyclohexenes.Chem Sci. 2015 Oct 1;6(10):5847-5852. doi: 10.1039/c5sc02379c. Epub 2015 Jul 10. Chem Sci. 2015. PMID: 29861910 Free PMC article.
-
Ring expansion and rearrangements of rhodium(II) azavinyl carbenes.Angew Chem Int Ed Engl. 2012 Dec 21;51(52):13054-7. doi: 10.1002/anie.201207820. Epub 2012 Nov 19. Angew Chem Int Ed Engl. 2012. PMID: 23161725 Free PMC article.
-
Dihalohydration of Alkynols: A Versatile Approach to Diverse Halogenated Molecules.European J Org Chem. 2018 Aug 7;2018(29):4018-4028. doi: 10.1002/ejoc.201800668. Epub 2018 Jul 18. European J Org Chem. 2018. PMID: 30147439 Free PMC article.
References
-
-
For selected examples of cyclobutene synthesis, see: Inanaga K, Takasu K, Ihara M. J. Am. Chem. Soc. 2005;127:3668. Liu YH, Liu MN, Song ZQ. J. Am. Chem. Soc. 2005;127:3662. Canales E, Corey EJ. J. Am. Chem. Soc. 2007;129:12686. Lopez-Carrillo V, Echavarren AM. J. Am. Chem. Soc. 2010;132:9292.
-
-
- Xu H, Zhang W, Shu D, Werness JB, Tang W. Angew. Chem. Int. Ed. 2008;47:8933. - PubMed
-
-
Um JM, Xu H, Houk KN, Tang W. J. Am. Chem. Soc. 2009;131:6664.; For related studies, see: Barluenga J, Riesgo L, Lopez LA, Rubio E, Tomas M. Angew. Chem. Int. Ed. 2009;48:7569. Li C-W, Pati K, Lin G-Y, Abu Sohel SM, Hung H-H, Liu R-S. Angew. Chem. Int. Ed. 2010;49:9891.
-
-
-
For selected reviews on cyclopropanations, see: Doyle MP. Chem. Rev. 1986;86:919. Ye T, McKervey MA. Chem. Rev. 1994;94:1091. Doyle MP, Forbes DC. Chem. Rev. 1998;98:911. Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. New York: John Wiley & Sons; 1998. Lebel H, Marcoux J-F, Molinaro C, Charette AB. Chem. Rev. 2003;103:977. Zhang Z, Wang J. Tetrahedron. 2008;64:6577. Pellissier H. Tetrahedron. 2008;64:7041. Davies HML, Denton JR. Chem. Soc. Rev. 2009;38:3061. Concellon JM, Rodriguez-Solla H, Concellon C, del Amo V. Chem. Soc. Rev. 2010;39:4103.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources