Spectroscopy and speciation studies on the interactions of aluminum (III) with ciprofloxacin and β-nicotinamide adenine dinucleotide phosphate in aqueous solutions
- PMID: 22864244
- PMCID: PMC6268657
- DOI: 10.3390/molecules17089379
Spectroscopy and speciation studies on the interactions of aluminum (III) with ciprofloxacin and β-nicotinamide adenine dinucleotide phosphate in aqueous solutions
Abstract
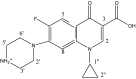
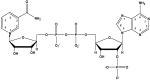
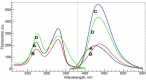
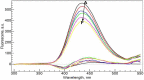
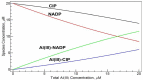
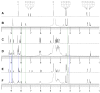
In this study, both experimental and theoretical approaches, including absorption spectra, fluorescence emission spectra, 1H- and 31P-NMR, electrospray ionization mass spectrometry (ESI-MS), pH-potentiometry and theoretical approaches using the BEST & SPE computer programs were applied to study the competitive complexation between ciprofloxacin (CIP) and b-nicotinamide adenine dinucleotide phosphate (NADP) with aluminum (III) in aqueous solutions. Rank annihilation factor analysis (RAFA) was used to analyze the absorption and fluorescence emission spectra of the ligands, the binary complexes and the ternary complexes. It is found, at the mM total concentration level and pH = 7.0, the bidentate mononuclear species [Al(CIP)]2+ and [Al(NADP)] predominate in the aqueous solutions of the Al(III)-CIP and Al(III)-NADP systems, and the two complexes have similar conditional stability constants. However, the pH-potentiometry results show at the mM total concentration level and pH = 7.0, the ternary species [Al(CIP)(HNADP)] predominates in the ternary complex system. Comparing predicted NMR spectra with the experimental NMR results, it can be concluded that for the ternary complex, CIP binds to aluminum ion between the 3-carboxylic and 4-carbonyl groups, while the binding site of oxidized coenzyme II is through the oxygen of phosphate, which is linked to adenosine ribose, instead of pyrophosphate. The results also suggested CIP has the potential to be a probe molecular for the detection of NADP and the Al(III)-NADP complexes under physiological condition.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

