Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts
- PMID: 22864415
- PMCID: PMC3443324
- DOI: 10.1038/nature11317
Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts
Abstract
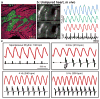
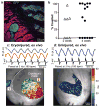
Transplantation studies in mice and rats have shown that human embryonic-stem-cell-derived cardiomyocytes (hESC-CMs) can improve the function of infarcted hearts, but two critical issues related to their electrophysiological behaviour in vivo remain unresolved. First, the risk of arrhythmias following hESC-CM transplantation in injured hearts has not been determined. Second, the electromechanical integration of hESC-CMs in injured hearts has not been demonstrated, so it is unclear whether these cells improve contractile function directly through addition of new force-generating units. Here we use a guinea-pig model to show that hESC-CM grafts in injured hearts protect against arrhythmias and can contract synchronously with host muscle. Injured hearts with hESC-CM grafts show improved mechanical function and a significantly reduced incidence of both spontaneous and induced ventricular tachycardia. To assess the activity of hESC-CM grafts in vivo, we transplanted hESC-CMs expressing the genetically encoded calcium sensor, GCaMP3 (refs 4, 5). By correlating the GCaMP3 fluorescent signal with the host ECG, we found that grafts in uninjured hearts have consistent 1:1 host–graft coupling. Grafts in injured hearts are more heterogeneous and typically include both coupled and uncoupled regions. Thus, human myocardial grafts meet physiological criteria for true heart regeneration, providing support for the continued development of hESC-based cardiac therapies for both mechanical and electrical repair.
Figures

References
-
- Caspi O, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. - PubMed
-
- Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. - PubMed
-
- van Laake LW, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Research. 2007;1:9–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL100405/HL/NHLBI NIH HHS/United States
- R01 HL084642/HL/NHLBI NIH HHS/United States
- R01 HL095828/HL/NHLBI NIH HHS/United States
- R01-HL064387/HL/NHLBI NIH HHS/United States
- P01-GM81619/GM/NIGMS NIH HHS/United States
- K08-HL80431/HL/NHLBI NIH HHS/United States
- P01 GM081619/GM/NIGMS NIH HHS/United States
- U24-DK076126/DK/NIDDK NIH HHS/United States
- P01 HL094374/HL/NHLBI NIH HHS/United States
- U24 DK076126/DK/NIDDK NIH HHS/United States
- R01-HL084642/HL/NHLBI NIH HHS/United States
- R01 HL064387/HL/NHLBI NIH HHS/United States
- R01-HL095828/HL/NHLBI NIH HHS/United States
- K08 HL080431/HL/NHLBI NIH HHS/United States
- P01-HL094374/HL/NHLBI NIH HHS/United States
- U01-HL100405/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

