The prokaryote messenger c-di-GMP triggers stalk cell differentiation in Dictyostelium
- PMID: 22864416
- PMCID: PMC3939355
- DOI: 10.1038/nature11313
The prokaryote messenger c-di-GMP triggers stalk cell differentiation in Dictyostelium
Abstract
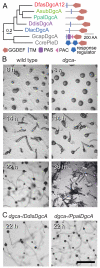
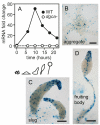
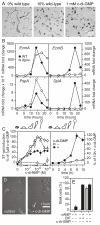
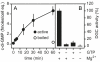
Cyclic di-(3′:5′)-guanosine monophosphate (c-di-GMP) is a major prokaryote signalling intermediate that is synthesized by diguanylate cyclases and triggers sessility and biofilm formation. We detected the first eukaryote diguanylate cyclases in all major groups of Dictyostelia. On food depletion, Dictyostelium discoideum amoebas collect into aggregates, which first transform into migrating slugs and then into sessile fruiting structures. These structures consist of a spherical spore mass that is supported by a column of stalk cells and a basal disk. A polyketide, DIF-1, which induces stalk-like cells in vitro, was isolated earlier. However, its role in vivo proved recently to be restricted to basal disk formation. Here we show that the Dictyostelium diguanylate cyclase, DgcA, produces c-di-GMP as the morphogen responsible for stalk cell differentiation. Dictyostelium discoideum DgcA synthesized c-di-GMP in a GTP-dependent manner and was expressed at the slug tip, which is the site of stalk cell differentiation. Disruption of the DgcA gene blocked the transition from slug migration to fructification and the expression of stalk genes. Fructification and stalk formation were restored by exposing DgcA-null slugs to wild-type secretion products or to c-di-GMP. Moreover, c-di-GMP, but not cyclic di-(3′:5′)-adenosine monophosphate, induced stalk gene expression in dilute cell monolayers. Apart from identifying the long-elusive stalk-inducing morphogen, our work also identifies a role for c-di-GMP in eukaryotes.
Figures
References
-
- Jenal U, Malone J. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu Rev Genet. 2006;40:385–407. - PubMed
-
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. - PubMed
-
- Morris HR, Taylor GW, Masento MS, Jermyn KA, Kay RR. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature. 1987;6133:811–814. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

