Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma
- PMID: 22864522
- PMCID: PMC4169197
- DOI: 10.1007/s10637-012-9862-2
Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma
Abstract
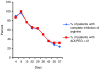
Background Arginine deiminase (ADI) is an enzyme that degrades arginine, an amino acid that is important for growth and development of normal and neoplastic cells. Melanoma cells are auxotrophic for arginine, because they lack argininosuccinatesynthetase (ASS), a key enzyme required for the synthesis of arginine. Patients and methods Patients with advanced melanoma were treated with 40, 80 or 160 IU/m(2) ADI-PEG 20 i.m. weekly. Primary endpoints were toxicity and tumor response, secondary endpoints included metabolic response by (18)FDG-PET, pharmacodynamic (PD) effects upon circulating arginine levels, and argininosuccinate synthetase tumor expression by immunohistochemistry. Results 31 previously treated patients were enrolled. The main toxicities were grade 1 and 2 adverse events including injection site pain, rash, and fatigue. No objective responses were seen. Nine patients achieved stable disease (SD), with 2 of these durable for >6 months. Four of the 9 patients with SD had uveal melanoma. PD analysis showed complete plasma arginine depletion in 30/31 patients by day 8. Mean plasma levels of ADI-PEG 20 correlated inversely with ADI-PEG 20 antibody levels. Immunohistochemical ASS expression analysis in tumor tissue was negative in 24 patients, whereas 5 patients had <5 % cells positive. Conclusions ADI-PEG 20 is well tolerated in advanced melanoma patients and leads to consistent, but transient, arginine depletion. Although no RECIST responses were observed, the encouraging rate of SD in uveal melanoma patients indicates that it may be worthwhile to evaluate ADI-PEG 20 in this melanoma subgroup.
Figures




References
-
- Albert VA, Koh HK, Geller AC, Miller DR, Prout MN, Lew RA. Years of potential life lost: another indicator of the impact of cutaneous malignant melanoma on society. J Am Acad Dermatol. 1990;23(2 Pt 1):308–310. - PubMed
-
- Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, Kirkwood JM, Haluska FG. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–4745. - PubMed
-
- Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, Begg CB, Agarwala SS, Schuchter LM, Ernstoff MS, Houghton AN, Kirkwood JM. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745–2751. - PubMed
-
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, Dreno B, Henz M, Schadendorf D, Kapp A, Weiss J, Fraass U, Statkevich P, Muller M, Thatcher N. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

