Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S
- PMID: 22864553
- PMCID: PMC3448907
- DOI: 10.1007/s00424-012-1138-3
Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S
Abstract
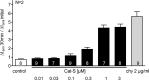
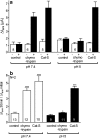
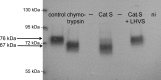
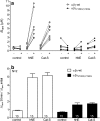
Proteolytic processing of the amiloride-sensitive epithelial sodium channel (ENaC) by serine proteases is known to be important for channel activation. Inappropriate ENaC activation by proteases may contribute to the pathophysiology of cystic fibrosis and could be involved in sodium retention and the pathogenesis of arterial hypertension in the context of renal disease. We hypothesized that in addition to serine proteases, cathepsin proteases may activate ENaC. Cathepsin proteases belong to the group of cysteine proteases and play a pathophysiological role in inflammatory diseases. Under pathophysiological conditions, cathepsin-S (Cat-S) may reach ENaC in the apical membrane of epithelial cells. The aim of this study was to investigate the effect of purified Cat-S on human ENaC heterologously expressed in Xenopus laevis oocytes and on ENaC-mediated sodium transport in cultured M-1 mouse renal collecting duct cells. We demonstrated that Cat-S activates amiloride-sensitive whole-cell currents in ENaC-expressing oocytes. The stimulatory effect of Cat-S was preserved at pH 5. ENaC stimulation by Cat-S was associated with the appearance of a γENaC cleavage fragment at the plasma membrane indicating proteolytic channel activation. Mutating two valine residues (V182 and V193) in the critical region of γENaC prevented proteolytic activation of ENaC by Cat-S. Pre-incubation of the oocytes with the Cat-S inhibitor morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl (LHVS) prevented the stimulatory effect of Cat-S on ENaC. In contrast, LHVS had no effect on ENaC activation by the prototypical serine proteases trypsin and chymotrypsin. Cat-S also stimulated ENaC in differentiated renal epithelial cells. These findings demonstrate that the cysteine protease Cat-S can activate ENaC which may be relevant under pathophysiological conditions.
Figures





References
-
- Ayesa S, Lindquist C, Agback T, Benkestock K, Classon B, Henderson I, Hewitt E, Jansson K, Kallin A, Sheppard D, Samuelsson B. Solid-phase parallel synthesis and SAR of 4-amidofuran-3-one inhibitors of cathepsin S: effect of sulfonamides P3 substituents on potency and selectivity. Bioorg Med Chem. 2009;17:1307–1324. doi: 10.1016/j.bmc.2008.12.020. - DOI - PubMed
-
- Bertog M, Letz B, Kong W, Steinhoff M, Higgins MA, Bielfeld Ackermann A, Frömter E, Bunnett NW, Korbmacher C. Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells. J Physiol. 1999;521:3–17. doi: 10.1111/j.1469-7793.1999.00003.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

