Interstitial cells in the primate gastrointestinal tract
- PMID: 22864981
- PMCID: PMC4806557
- DOI: 10.1007/s00441-012-1468-7
Interstitial cells in the primate gastrointestinal tract
Abstract
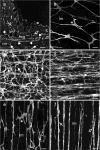
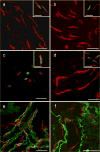
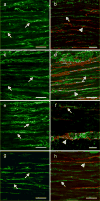
Kit immunohistochemistry and confocal reconstructions have provided detailed 3-dimensional images of ICC networks throughout the gastrointestinal (GI) tract. Morphological criteria have been used to establish that different classes of ICC exist within the GI tract and physiological studies have shown that these classes have distinct physiological roles in GI motility. Structural studies have focused predominately on rodent models and less information is available on whether similar classes of ICC exist within the GI tracts of humans or non-human primates. Using Kit immunohistochemistry and confocal imaging, we examined the 3-dimensional structure of ICC throughout the GI tract of cynomolgus monkeys. Whole or flat mounts and cryostat sections were used to examine ICC networks in the lower esophageal sphincter (LES), stomach, small intestine and colon. Anti-histamine antibodies were used to distinguish ICC from mast cells in the lamina propria. Kit labeling identified complex networks of ICC populations throughout the non-human primate GI tract that have structural characteristics similar to that described for ICC populations in rodent models. ICC-MY formed anastomosing networks in the myenteric plexus region. ICC-IM were interposed between smooth muscle cells in the stomach and colon and were concentrated within the deep muscular plexus (ICC-DMP) of the intestine. ICC-SEP were found in septal regions of the antrum that separated circular muscle bundles. Spindle-shaped histamine(+) mast cells were found in the lamina propria throughout the GI tract. Since similar sub-populations of ICC exist within the GI tract of primates and rodents and the use of rodents to study the functional roles of different classes of ICC is warranted.
Figures




References
-
- Albertí E, Mikkelsen HB, Wang XY, Díaz M, Larsen JO, Huizinga JD, Jiménez M. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–1510. - PubMed
-
- Aranishi H, Kunisawa Y, Komuro T. Characterization of interstitial cells of Cajal in the subserosal layer of the guinea-pig colon. Cell Tissue Res. 2009;335:323–329. - PubMed
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

