Study of transforming growth factor alpha for the maintenance of human embryonic stem cells
- PMID: 22864984
- PMCID: PMC3480587
- DOI: 10.1007/s00441-012-1476-7
Study of transforming growth factor alpha for the maintenance of human embryonic stem cells
Abstract
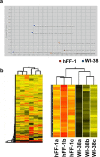
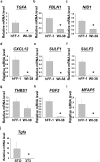
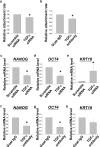
Human embryonic stem cells (hESCs) have great potential for regenerative medicine as they have self-regenerative and pluripotent properties. Feeder cells or their conditioned medium are required for the maintenance of hESC in the undifferentiated state. Feeder cells have been postulated to produce growth factors and extracellular molecules for maintaining hESC in culture. The present study has aimed at identifying these molecules. The gene expression of supportive feeder cells, namely human foreskin fibroblast (hFF-1) and non-supportive human lung fibroblast (WI-38) was analyzed by microarray and 445 genes were found to be differentially expressed. Gene ontology analysis showed that 20.9% and 15.5% of the products of these genes belonged to the extracellular region and regulation of transcription activity, respectively. After validation of selected differentially expressed genes in both human and mouse feeder cells, transforming growth factor α (TGFα) was chosen for functional study. The results demonstrated that knockdown or protein neutralization of TGFα in hFF-1 led to increased expression of early differentiation markers and lower attachment rates of hESC. More importantly, TGFα maintained pluripotent gene expression levels, attachment rates and pluripotency by the in vitro differentiation of H9 under non-supportive conditions. TGFα treatment activated the p44/42 MAPK pathway but not the PI3K/Akt pathway. In addition, TGFα treatment increased the expression of pluripotent markers, NANOG and SSEA-3 but had no effects on the proliferation of hESCs. This study of the functional role of TGFα provides insights for the development of clinical grade hESCs for therapeutic applications.
Figures




Similar articles
-
Laminin-511 expression is associated with the functionality of feeder cells in human embryonic stem cell culture.Stem Cell Res. 2012 Jan;8(1):97-108. doi: 10.1016/j.scr.2011.08.005. Epub 2011 Aug 27. Stem Cell Res. 2012. PMID: 22099024
-
Maintenance of human embryonic stem cells in media conditioned by human mesenchymal stem cells obviates the requirement of exogenous basic fibroblast growth factor supplementation.Tissue Eng Part C Methods. 2012 May;18(5):387-96. doi: 10.1089/ten.TEC.2011.0546. Epub 2012 Jan 26. Tissue Eng Part C Methods. 2012. PMID: 22136131
-
Establishment of clinically compliant human embryonic stem cells in an autologous feeder-free system.Tissue Eng Part C Methods. 2011 Sep;17(9):927-37. doi: 10.1089/ten.TEC.2010.0735. Epub 2011 Jun 20. Tissue Eng Part C Methods. 2011. PMID: 21561302
-
Embryonic stem cells: isolation, characterization and culture.Adv Biochem Eng Biotechnol. 2009;114:173-84. doi: 10.1007/10_2008_20. Adv Biochem Eng Biotechnol. 2009. PMID: 19495683 Review.
-
A review of gene expression profiling of human embryonic stem cell lines and their differentiated progeny.Curr Stem Cell Res Ther. 2009 May;4(2):98-106. doi: 10.2174/157488809788167409. Curr Stem Cell Res Ther. 2009. PMID: 19442194 Review.
Cited by
-
Infection and Functional Modulation of Human Monocytes and Macrophages by Varicella-Zoster Virus.J Virol. 2019 Jan 17;93(3):e01887-18. doi: 10.1128/JVI.01887-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30404793 Free PMC article.
-
CBARA1 plays a role in stemness and proliferation of human embryonic stem cells.PLoS One. 2013 May 8;8(5):e63653. doi: 10.1371/journal.pone.0063653. Print 2013. PLoS One. 2013. PMID: 23667653 Free PMC article.
-
Different transcriptional profiles of human embryonic stem cells grown in a feeder-free culture system and on human foreskin fibroblast feeder layers.Aging (Albany NY). 2022 Sep 13;14(18):7443-7454. doi: 10.18632/aging.204282. Epub 2022 Sep 13. Aging (Albany NY). 2022. PMID: 36103219 Free PMC article.
-
From wavy hair to naked proteins: the role of transforming growth factor alpha in health and disease.Semin Cell Dev Biol. 2014 Apr;28:12-21. doi: 10.1016/j.semcdb.2014.03.003. Epub 2014 Mar 12. Semin Cell Dev Biol. 2014. PMID: 24631356 Free PMC article. Review.
References
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. - DOI - PubMed
-
- Catalina P, Montes R, Ligero G, Sanchez L, de la Cueva T, Bueno C, Leone PE, Menendez P. Human ESCs predisposition to karyotypic instability: is a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol Cancer. 2008;7:76. doi: 10.1186/1476-4598-7-76. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

