Memory T cells are uniquely resistant to melanoma-induced suppression
- PMID: 22865267
- PMCID: PMC4550291
- DOI: 10.1007/s00262-012-1326-1
Memory T cells are uniquely resistant to melanoma-induced suppression
Abstract
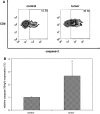
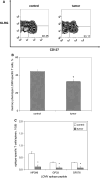
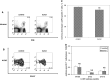
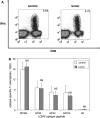
We have previously observed that in vivo exposure to growing melanoma tumors fundamentally alters activated T cell homeostasis by suppressing the ability of naïve T cells to undergo antigen-driven proliferative expansion. We hypothesized that exposure of T cells in later stages of differentiation to melanoma would have similar suppressive consequences. C57BL/6 mice were inoculated with media or syngeneic B16F10 melanoma tumors 8 or 60 days after infection with lymphocytic choriomeningitis virus (LCMV), and splenic populations of LCMV-specific T cells were quantified using flow cytometry 18 days after tumor inoculation. Inoculation with melanoma on post-infection day 8 potentiated the contraction of previously activated T cells. This enhanced contraction was associated with increased apoptotic susceptibility among T cells from tumor-bearing mice. In contrast, inoculation with melanoma on post-infection day 60 did not affect the ability of previously established memory T cells to maintain themselves in stable numbers. In addition, the ability of previously established memory T cells to respond to LCMV challenge was unaffected by melanoma. Following adoptive transfer into melanoma-bearing mice, tumor-specific memory T cells were significantly more effective at controlling melanoma growth than equivalent numbers of tumor-specific effector T cells. These observations suggest that memory T cells are uniquely resistant to suppressive influences exerted by melanoma on activated T cell homeostasis; these findings may have implications for T cell-based cancer immunotherapy.
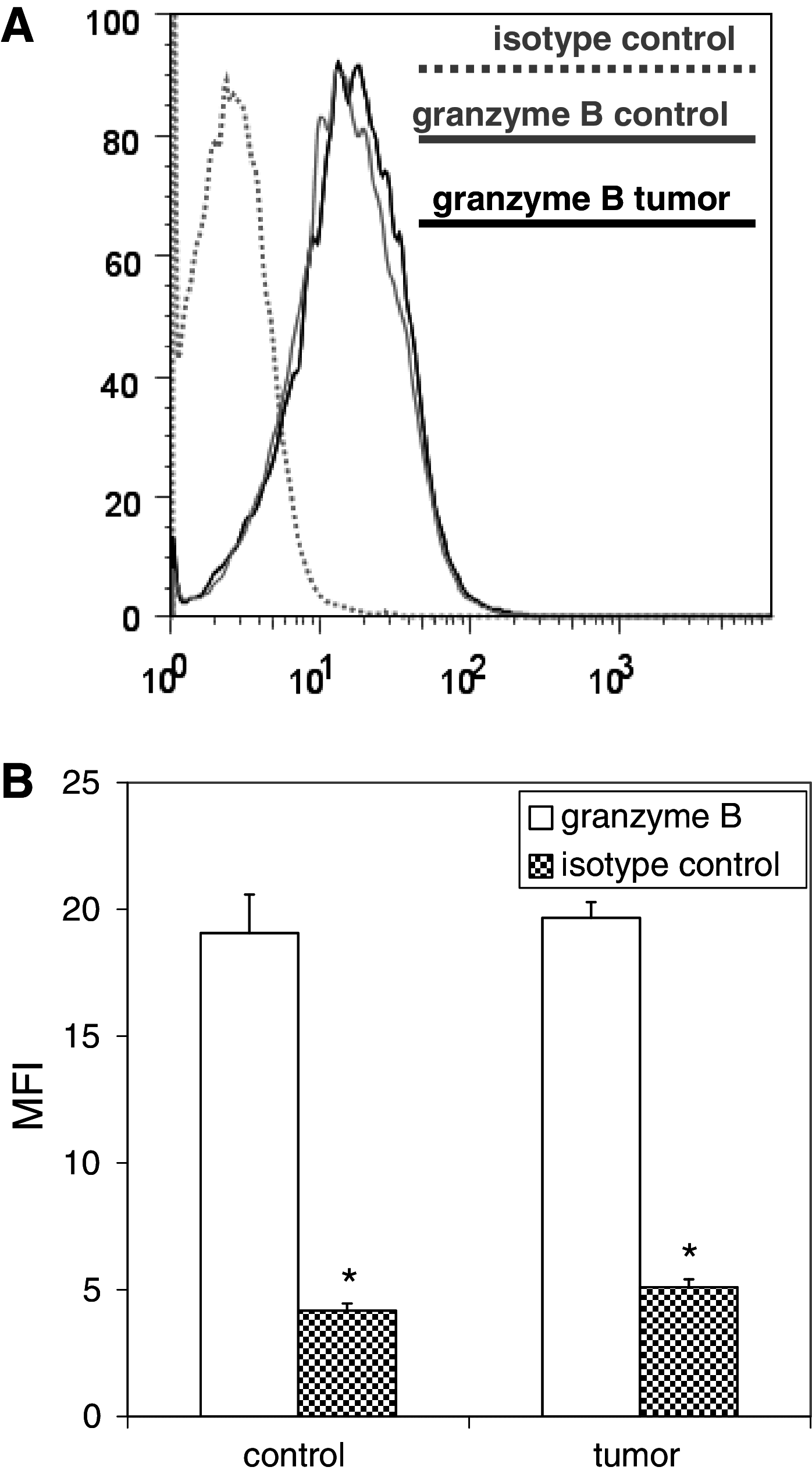
Figures



Similar articles
-
Co-transfer of tumor-specific effector and memory CD8+ T cells enhances the efficacy of adoptive melanoma immunotherapy in a mouse model.J Immunother Cancer. 2018 May 29;6(1):41. doi: 10.1186/s40425-018-0358-2. J Immunother Cancer. 2018. PMID: 29843822 Free PMC article.
-
Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice.Cancer Immunol Immunother. 2016 May;65(5):601-11. doi: 10.1007/s00262-016-1823-8. Epub 2016 Mar 24. Cancer Immunol Immunother. 2016. PMID: 27011014 Free PMC article.
-
Suppression of T-cell expansion by melanoma is exerted on resting cells.Ann Surg Oncol. 2011 Dec;18(13):3848-57. doi: 10.1245/s10434-011-1667-6. Epub 2011 Apr 5. Ann Surg Oncol. 2011. PMID: 21465311 Free PMC article.
-
Melanoma-induced suppression of tumor antigen-specific T cell expansion is comparable to suppression of global T cell expansion.Cell Immunol. 2011;271(1):104-9. doi: 10.1016/j.cellimm.2011.06.011. Epub 2011 Jun 17. Cell Immunol. 2011. PMID: 21741629 Free PMC article.
-
Kinetics of the response of naive and memory CD8 T cells to antigen: similarities and differences.Eur J Immunol. 1999 Jan;29(1):284-90. doi: 10.1002/(SICI)1521-4141(199901)29:01<284::AID-IMMU284>3.0.CO;2-C. Eur J Immunol. 1999. PMID: 9933110
Cited by
-
Co-transfer of tumor-specific effector and memory CD8+ T cells enhances the efficacy of adoptive melanoma immunotherapy in a mouse model.J Immunother Cancer. 2018 May 29;6(1):41. doi: 10.1186/s40425-018-0358-2. J Immunother Cancer. 2018. PMID: 29843822 Free PMC article.
-
Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation.Front Immunol. 2023 Jan 23;14:1012799. doi: 10.3389/fimmu.2023.1012799. eCollection 2023. Front Immunol. 2023. PMID: 36756111 Free PMC article.
-
Ctla-4 blockade plus adoptive T-cell transfer promotes optimal melanoma immunity in mice.J Immunother. 2015 Feb-Mar;38(2):54-61. doi: 10.1097/CJI.0000000000000064. J Immunother. 2015. PMID: 25658614 Free PMC article.
-
Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice.Cancer Immunol Immunother. 2016 May;65(5):601-11. doi: 10.1007/s00262-016-1823-8. Epub 2016 Mar 24. Cancer Immunol Immunother. 2016. PMID: 27011014 Free PMC article.
-
Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy.J Immunother Cancer. 2020 Jan;8(1):e000200. doi: 10.1136/jitc-2019-000200. J Immunother Cancer. 2020. PMID: 31940590 Free PMC article.
References
-
- Sarkar S, Teichgräber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

