Direct activation of Xenopus iodotyrosine deiodinase by thyroid hormone receptor in the remodeling intestine during amphibian metamorphosis
- PMID: 22865369
- PMCID: PMC3512013
- DOI: 10.1210/en.2012-1308
Direct activation of Xenopus iodotyrosine deiodinase by thyroid hormone receptor in the remodeling intestine during amphibian metamorphosis
Abstract
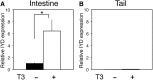
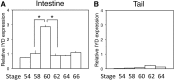
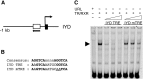
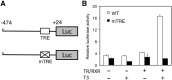
Thyroid hormone (TH) plays critical roles during vertebrate postembryonic development. TH production in the thyroid involves incorporating inorganic iodide into thyroglobulin. The expression of iodotyrosine deiodinase (IYD; also known as iodotyrosine dehalogenase 1) in the thyroid gland ensures efficient recycling of iodine from the byproducts of TH biosynthesis: 3'-monoiodotyrosine and 3', 5'-diiodotyrosine. Interestingly, IYD is known to be expressed in other organs in adult mammals, suggesting iodine recycling outside the thyroid. On the other hand, the developmental role of iodine recycling has yet to be investigated. Here, using intestinal metamorphosis as a model, we discovered that the Xenopus tropicalis IYD gene is strongly up-regulated by TH during metamorphosis in the intestine but not the tail. We further demonstrated that this induction was one of the earliest events during intestinal metamorphosis, with IYD being activated directly through the binding of liganded TH receptors to a TH response element in the IYD promoter region. Because iodide is mainly taken up from the diet in the intestine and the tadpole stops feeding during metamorphosis when the intestine is being remodeled, our findings suggest that IYD transcription is activated by liganded TH receptors early during intestinal remodeling to ensure efficient iodine recycling at the climax of metamorphosis when highest levels of TH are needed for the proper transformations of different organs.
Figures


Similar articles
-
Evaluating Iodide Recycling Inhibition as a Novel Molecular Initiating Event for Thyroid Axis Disruption in Amphibians.Toxicol Sci. 2018 Dec 1;166(2):318-331. doi: 10.1093/toxsci/kfy203. Toxicol Sci. 2018. PMID: 30137636 Free PMC article.
-
Timing of metamorphosis and the onset of the negative feedback loop between the thyroid gland and the pituitary is controlled by type II iodothyronine deiodinase in Xenopus laevis.Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7348-53. doi: 10.1073/pnas.131198998. Epub 2001 Jun 12. Proc Natl Acad Sci U S A. 2001. PMID: 11404476 Free PMC article.
-
Lamprey metamorphosis: Thyroid hormone signaling in a basal vertebrate.Mol Cell Endocrinol. 2017 Dec 25;459:28-42. doi: 10.1016/j.mce.2017.06.015. Epub 2017 Jun 16. Mol Cell Endocrinol. 2017. PMID: 28630022 Review.
-
Thyroid Hormone Receptor Is Essential for Larval Epithelial Apoptosis and Adult Epithelial Stem Cell Development but Not Adult Intestinal Morphogenesis during Xenopus tropicalis Metamorphosis.Cells. 2021 Mar 3;10(3):536. doi: 10.3390/cells10030536. Cells. 2021. PMID: 33802526 Free PMC article.
-
Xenopus metamorphosis as a model to study thyroid hormone receptor function during vertebrate developmental transitions.Mol Cell Endocrinol. 2017 Dec 25;459:64-70. doi: 10.1016/j.mce.2017.03.020. Epub 2017 Mar 28. Mol Cell Endocrinol. 2017. PMID: 28363743 Review.
Cited by
-
Differential regulation of two histidine ammonia-lyase genes during Xenopus development implicates distinct functions during thyroid hormone-induced formation of adult stem cells.Cell Biosci. 2013 Nov 13;3(1):43. doi: 10.1186/2045-3701-3-43. Cell Biosci. 2013. PMID: 24499573 Free PMC article.
-
Cytological and morphological analyses reveal distinct features of intestinal development during Xenopus tropicalis metamorphosis.PLoS One. 2012;7(10):e47407. doi: 10.1371/journal.pone.0047407. Epub 2012 Oct 10. PLoS One. 2012. PMID: 23071801 Free PMC article.
-
A balance of Mad and Myc expression dictates larval cell apoptosis and adult stem cell development during Xenopus intestinal metamorphosis.Cell Death Dis. 2017 May 11;8(5):e2787. doi: 10.1038/cddis.2017.198. Cell Death Dis. 2017. PMID: 28492553 Free PMC article.
-
Cross-species comparison of chemical inhibition of human and Xenopus iodotyrosine deiodinase.Aquat Toxicol. 2022 Aug;249:106227. doi: 10.1016/j.aquatox.2022.106227. Epub 2022 Jun 15. Aquat Toxicol. 2022. PMID: 35767922 Free PMC article.
-
Histone methyltransferase Dot1L is a coactivator for thyroid hormone receptor during Xenopus development.FASEB J. 2017 Nov;31(11):4821-4831. doi: 10.1096/fj.201700131R. Epub 2017 Jul 24. FASEB J. 2017. PMID: 28739643 Free PMC article.
References
-
- Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 - PubMed
-
- Hetzel BS. 1989. The story of iodine deficiency: an international challenge in nutrition. Oxford, UK: Oxford University Press
-
- Jhiang SM. 2000. Regulation of sodium/iodide symporter. Rev Endocr Metab Disord 1:205–215 - PubMed
-
- Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. 2003. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 24:48–77 - PubMed
-
- Moreno JC, Visser TJ. 2010. Genetics and phenomics of hypothyroidism and goiter due to iodotyrosine deiodinase (DEHAL1) gene mutations. Mol Cell Endocrinol 322:91–98 - PubMed

