Roles for endothelin receptor B and BCL2A1 in spontaneous CNS metastasis of melanoma
- PMID: 22865454
- PMCID: PMC4334445
- DOI: 10.1158/0008-5472.CAN-12-2194
Roles for endothelin receptor B and BCL2A1 in spontaneous CNS metastasis of melanoma
Abstract
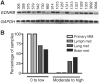
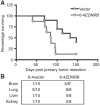
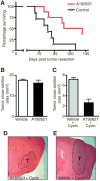
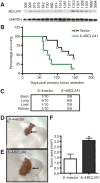
Metastatic spread of melanoma to the central nervous system (CNS) is a common and devastating manifestation of disease progression, which, despite its clinical importance, remains poorly understood with respect to underlying molecular mechanisms. Using a recently developed preclinical model of spontaneous melanoma CNS metastasis, we have identified alterations in expression of endothelin receptor B (EDNRB) as a potential factor that influences brain metastatic potential. Induced overexpression of this gene mediated enhanced overall metastatic disease, and resulted in an increased incidence of spontaneous CNS metastases. In contrast, the overexpression of other highlighted genes, such as BCL2A1, did not affect the incidence of CNS metastases but nevertheless appears to facilitate intracranial tumor growth. The prometastatic effect in the CNS associated with EDNRB appears to be mediated by the interaction with its ligands resulting in enhanced tumor cell proliferation and thus intracranial melanoma growth. That EDNRB contributes to melanoma metastasis is underscored by the fact that its therapeutic inhibition by the EDNRB-specific inhibitor A192621 translated into improved outcomes when treating mice with either visceral metastases or intracranial tumors. The identification of an influential role of EDNRB in CNS melanoma spontaneous metastasis may provide both a target for therapeutic intervention as well as a potential prognostic marker for patients having an increased predisposition for incidence of CNS melanoma metastases.
©2012 AACR.
Figures

Similar articles
-
First-in-Human Proof-of-Concept Study: Intralesional Administration of BQ788, an Endothelin Receptor B Antagonist, to Melanoma Skin Metastases.Oncologist. 2015 Oct;20(10):1121-2. doi: 10.1634/theoncologist.2015-0139. Epub 2015 Sep 1. Oncologist. 2015. PMID: 26330458 Free PMC article. Clinical Trial.
-
Endothelin receptor B antagonists decrease glioma cell viability independently of their cognate receptor.BMC Cancer. 2008 Nov 28;8:354. doi: 10.1186/1471-2407-8-354. BMC Cancer. 2008. PMID: 19040731 Free PMC article.
-
MAPK pathway inhibition enhances the efficacy of an anti-endothelin B receptor drug conjugate by inducing target expression in melanoma.Mol Cancer Ther. 2014 Jun;13(6):1599-610. doi: 10.1158/1535-7163.MCT-13-0446. Epub 2014 Mar 20. Mol Cancer Ther. 2014. PMID: 24651527
-
Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma.Int J Dev Biol. 2005;49(2-3):173-80. doi: 10.1387/ijdb.041951rl. Int J Dev Biol. 2005. PMID: 15906230 Review.
-
Molecular testing and targeting for solid tumors with CNS metastases.J Neurooncol. 2025 May;173(1):1-10. doi: 10.1007/s11060-025-04947-9. Epub 2025 Apr 3. J Neurooncol. 2025. PMID: 40178767 Review.
Cited by
-
Clinical Significance of PDCD4 in Melanoma by Subcellular Expression and in Tumor-Associated Immune Cells.Cancers (Basel). 2021 Mar 2;13(5):1049. doi: 10.3390/cancers13051049. Cancers (Basel). 2021. PMID: 33801444 Free PMC article.
-
Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells.Neuro Oncol. 2014 Dec;16(12):1585-98. doi: 10.1093/neuonc/nou128. Epub 2014 Jul 9. Neuro Oncol. 2014. PMID: 25008093 Free PMC article.
-
Hypermethylation of EDNRB promoter contributes to the risk of colorectal cancer.Diagn Pathol. 2013 Dec 10;8:199. doi: 10.1186/1746-1596-8-199. Diagn Pathol. 2013. PMID: 24326135 Free PMC article.
-
Treatment of experimental human breast cancer and lung cancer brain metastases in mice by macitentan, a dual antagonist of endothelin receptors, combined with paclitaxel.Neuro Oncol. 2016 Apr;18(4):486-96. doi: 10.1093/neuonc/now037. Neuro Oncol. 2016. PMID: 26995790 Free PMC article.
-
Role of the blood-brain barrier in the formation of brain metastases.Int J Mol Sci. 2013 Jan 11;14(1):1383-411. doi: 10.3390/ijms14011383. Int J Mol Sci. 2013. PMID: 23344048 Free PMC article. Review.
References
-
- Maher EA, Mietz J, Arteaga CL, DePinho RA, Mohla S. Brain metastasis: opportunities in basic and translational research. Cancer Res. 2009;69:6015–20. - PubMed
-
- Tarhini AA, Agarwala SS. Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. - PubMed
-
- Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat Rev. 2004;30:515–20. - PubMed
-
- Vultur A, Villanueva J, Herlyn M. BRAF inhibitor unveils its potential against advanced melanoma. Cancer Cell. 2010;18:301–2. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

