NFAT1 supports tumor-induced anergy of CD4(+) T cells
- PMID: 22865456
- PMCID: PMC3445721
- DOI: 10.1158/0008-5472.CAN-11-3775
NFAT1 supports tumor-induced anergy of CD4(+) T cells
Abstract
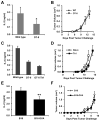
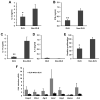
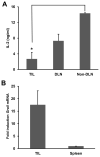
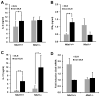
Cancer cells express antigens that elicit T cell-mediated responses, but these responses are limited during malignant progression by the development of immunosuppressive mechanisms in the tumor microenvironment that drive immune escape. T-cell hyporesponsiveness can be caused by clonal anergy or adaptive tolerance, but the pathophysiological roles of these processes in specific tumor contexts has yet to be understood. In CD4+ T cells, clonal anergy occurs when the T-cell receptor is activated in the absence of a costimulatory signal. Here we report that the key T-cell transcription factor NFAT mediates expression of anergy-associated genes in the context of cancer. Specifically, in a murine model of melanoma, we found that cancer cells induced anergy in antigen-specific CD4+ T-cell populations, resulting in defective production of several key effector cytokines. NFAT1 deficiency blunted the induction of anergy in tumor antigen-specific CD4+ T cells, enhancing antitumor responses. These investigations identified tumor-induced T-cell hyporesponsiveness as a form of clonal anergy, and they supported an important role for CD4+ T-cell anergy in driving immune escape. By illustrating the dependence of tumor-induced CD4+ T-cell anergy on NFAT1, our findings open the possibility of targeting this transcription factor to improve the efficacy of cancer immunotherapy or immunochemotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–66. - PubMed
-
- Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309–16. - PubMed
-
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

