Factors that differentiate the H-bond strengths of water near the Schiff bases in bacteriorhodopsin and Anabaena sensory rhodopsin
- PMID: 22865888
- PMCID: PMC3464511
- DOI: 10.1074/jbc.M112.388348
Factors that differentiate the H-bond strengths of water near the Schiff bases in bacteriorhodopsin and Anabaena sensory rhodopsin
Abstract
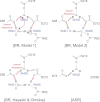
Bacteriorhodopsin (BR) functions as a light-driven proton pump, whereas Anabaena sensory rhodopsin (ASR) is believed to function as a photosensor despite the high similarity in their protein sequences. In Fourier transform infrared (FTIR) spectroscopic studies, the lowest O-D stretch for D(2)O was observed at ∼2200 cm(-1) in BR but was significantly higher in ASR (>2500 cm(-1)), which was previously attributed to a water molecule near the Schiff base (W402) that is H-bonded to Asp-85 in BR and Asp-75 in ASR. We investigated the factors that differentiate the lowest O-D stretches of W402 in BR and ASR. Quantum mechanical/molecular mechanical calculations reproduced the H-bond geometries of the crystal structures, and the calculated O-D stretching frequencies were corroborated by the FTIR band assignments. The potential energy profiles indicate that the smaller O-D stretching frequency in BR originates from the significantly higher pK(a)(Asp-85) in BR relative to the pK(a)(Asp-75) in ASR, which were calculated to be 1.5 and -5.1, respectively. The difference is mostly due to the influences of Ala-53, Arg-82, Glu-194-Glu-204, and Asp-212 on pK(a)(Asp-85) in BR and the corresponding residues Ser-47, Arg-72, Ser-188-Asp-198, and Pro-206 on pK(a)(Asp-75) in ASR. Because these residues participate in proton transfer pathways in BR but not in ASR, the presence of a strongly H-bonded water molecule near the Schiff base ultimately results from the proton-pumping activity in BR.
Figures

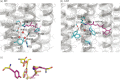



Similar articles
-
FTIR spectroscopy of the all-trans form of Anabaena sensory rhodopsin at 77 K: hydrogen bond of a water between the Schiff base and Asp75.Biochemistry. 2005 Sep 20;44(37):12287-96. doi: 10.1021/bi050841o. Biochemistry. 2005. PMID: 16156642
-
Hydrogen-bonding interaction of the protonated schiff base with halides in a chloride-pumping bacteriorhodopsin mutant.Biochemistry. 2006 Sep 5;45(35):10633-40. doi: 10.1021/bi060555s. Biochemistry. 2006. PMID: 16939215
-
FTIR study of the photoisomerization processes in the 13-cis and all-trans forms of Anabaena sensory rhodopsin at 77 K.Biochemistry. 2006 Apr 11;45(14):4362-70. doi: 10.1021/bi052324b. Biochemistry. 2006. PMID: 16584171
-
Hydration switch model for the proton transfer in the Schiff base region of bacteriorhodopsin.Biochim Biophys Acta. 2004 Jul 23;1658(1-2):72-9. doi: 10.1016/j.bbabio.2004.03.015. Biochim Biophys Acta. 2004. PMID: 15282177 Review.
-
Atomic resolution structures of bacteriorhodopsin photocycle intermediates: the role of discrete water molecules in the function of this light-driven ion pump.Biochim Biophys Acta. 2000 Aug 30;1460(1):133-56. doi: 10.1016/s0005-2728(00)00135-3. Biochim Biophys Acta. 2000. PMID: 10984596 Review.
Cited by
-
Combined Mutational and Spectroscopic Study on the Calcium-Related Kinetic Effects on the VirChR1 Photocycle.J Phys Chem B. 2025 Mar 20;129(11):2946-2957. doi: 10.1021/acs.jpcb.4c08416. Epub 2025 Mar 10. J Phys Chem B. 2025. PMID: 40063977 Free PMC article.
-
Vectorial Proton Transport Mechanism of RxR, a Phylogenetically Distinct and Thermally Stable Microbial Rhodopsin.Sci Rep. 2020 Jan 14;10(1):282. doi: 10.1038/s41598-019-57122-2. Sci Rep. 2020. PMID: 31937866 Free PMC article.
-
Correlation between C═O Stretching Vibrational Frequency and pKa Shift of Carboxylic Acids.J Phys Chem B. 2022 Jul 14;126(27):4999-5006. doi: 10.1021/acs.jpcb.2c02193. Epub 2022 Jun 28. J Phys Chem B. 2022. PMID: 35763701 Free PMC article.
-
X-ray structure analysis of bacteriorhodopsin at 1.3 Å resolution.Sci Rep. 2018 Sep 3;8(1):13123. doi: 10.1038/s41598-018-31370-0. Sci Rep. 2018. PMID: 30177765 Free PMC article.
-
A natural light-driven inward proton pump.Nat Commun. 2016 Nov 17;7:13415. doi: 10.1038/ncomms13415. Nat Commun. 2016. PMID: 27853152 Free PMC article.
References
-
- Lanyi J. K. (1998) Understanding structure and function in the light-driven proton pump bacteriorhodopsin. J. Struct. Biol. 124, 164–178 - PubMed
-
- Balashov S. P. (2000) Protonation reactions and their coupling in bacteriorhodopsin. Biochim. Biophys. Acta 1460, 75–94 - PubMed
-
- Kandori H. (2000) Role of internal water molecules in bacteriorhodopsin. Biochim. Biophys. Acta 1460, 177–191 - PubMed
-
- Maeda A., Sasaki J., Yamazaki Y., Needleman R., Lanyi J. K. (1994) Interaction of aspartate-85 with a water molecule and the protonated Schiff base in the L intermediate of bacteriorhodopsin. A Fourier-transform infrared spectroscopic study. Biochemistry 33, 1713–1717 - PubMed
-
- Kandori H., Yamazaki Y., Sasaki J., Needleman R., Lanyi J. K., Maeda A. (1995) Water-mediated proton transfer in proteins. An FTIR study of bacteriorhodopsin. J. Am. Chem. Soc. 117, 2118–2119

