Effect of tumour necrosis factor-alpha on estrogen metabolic pathways in breast cancer cells
- PMID: 22866165
- PMCID: PMC3408695
- DOI: 10.7150/jca.4584
Effect of tumour necrosis factor-alpha on estrogen metabolic pathways in breast cancer cells
Abstract
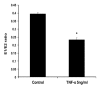
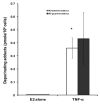
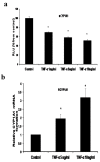
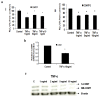
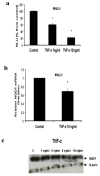
Tumor necrosis factor-alpha (TNF-α) is a proinflammatory cytokine that has been linked to breast cancer development. Estrogen metabolic pathway is also involved in breast carcinogenesis and DNA adducts formation. In this study we investigated the effect of TNF-α on the estrogen metabolic pathway in MCF-7, a breast cancer cell line. Capillary liquid chromatography/mass spectrometry (LC/MS) and High performance liquid chromatography (HPLC) were used for analysis of estrogen metabolites and estrogen-DNA adducts levels respectively. Reporter gene assay, Real time reverse transcription polymerase chain reaction (real time RT-PCR) and Western blot were used to assess the expression of estrogen metabolizing genes and enzymes. TNF-α significantly increased the total EM and decreased the estrone (E1) / 17-β estradiol (E2) ratio. Moreover, it altered the expression of genes and enzymes involved in E2 activation and deactivation pathways e.g. Cytochrome P-450 1A1 (CYP1A1), Cytochrome P-450 1B1 (CYP1B1), Catechol-O-methyl transferase (COMT) and Nicotinamide adenine dinucleotide phosphate-quinone oxidoreductase 1 (NQO1). In addition, there were increased levels of some catechol estrogens e.g. 4-hydroxy-estrone (4-OHE1) and 2-hydroxyestradiol (2-OHE2) with decreased levels of methylated catechols e.g. 2-methoxy estradiol (2-MeOE2). DNA adducts especially 4-OHE1-[2]-1-N3 Adenine was significantly increased. TNF-α directs the estrogen metabolism into more hormonally active and carcinogenic products in MCF-7. This may implicate a new possible explanation for inflammation associated breast cancer.
Keywords: Breast cancer; DNA adducts.; Estrogen metabolites; Estrogen metabolizing genes and enzymes; Tumor necrosis factor-alpha.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61(6):409–18. - PubMed
-
- Henderson IC, Canellos GP. Cancer of the breast: the past decade. N Engl J Med. 1990;302:17–30. - PubMed
-
- Henderson HS, Ross RK, Bernstein L. Estrogens as a cause of human cancer: The Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1988;48:246–53. - PubMed
-
- Russo j, Russo IH. Cellular basis of breast cancer susceptibility. Oncol Res. 1999;11(4):169–78. - PubMed
-
- Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–334. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

