Micro-ultrasound for preclinical imaging
- PMID: 22866232
- PMCID: PMC3262267
- DOI: 10.1098/rsfs.2011.0037
Micro-ultrasound for preclinical imaging
Abstract
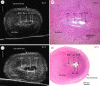
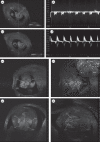
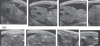
Over the past decade, non-invasive preclinical imaging has emerged as an important tool to facilitate biomedical discovery. Not only have the markets for these tools accelerated, but the numbers of peer-reviewed papers in which imaging end points and biomarkers have been used have grown dramatically. High frequency 'micro-ultrasound' has steadily evolved in the post-genomic era as a rapid, comparatively inexpensive imaging tool for studying normal development and models of human disease in small animals. One of the fundamental barriers to this development was the technological hurdle associated with high-frequency array transducers. Recently, new approaches have enabled the upper limits of linear and phased arrays to be pushed from about 20 to over 50 MHz enabling a broad range of new applications. The innovations leading to the new transducer technology and scanner architecture are reviewed. Applications of preclinical micro-ultrasound are explored for developmental biology, cancer, and cardiovascular disease. With respect to the future, the latest developments in high-frequency ultrasound imaging are described.
Keywords: angiogenesis; cancer models; cardiovascular disease; micro-ultrasound; mouse imaging; phenotyping.
Figures



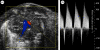
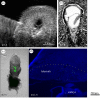
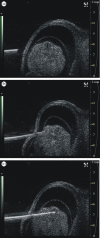
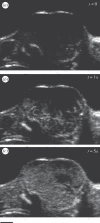
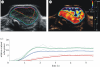
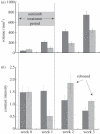
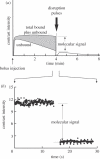



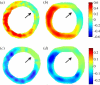
References
-
- Frost & Sullivan 2010. US preclinical animal imaging systems market. N5BB-50, 1–103
-
- Sherar M. D., Noss M. B., Foster F. S. 1987. Ultrasound backscatter microscopy images the internal structure of living tumour spheroids. Nature 330, 493–495 10.1038/330493a0 (doi:10.1038/330493a0) - DOI - PubMed
-
- Pavlin C. J., Harasiewicz K., Sherar M. D., Foster F. S. 1991. Clinical use of ultrasound biomicroscopy. Ophthalmology 98, 287–295 - PubMed
-
- Pavlin C. J., Sherar M. D., Foster F. S. 1990. Subsurface imaging of the eye by ultrasound biomicroscopy. Ophthalmology 97, 244–250 - PubMed
-
- Sherar M. D., Starkoski B. G., Taylor W. B., Foster F. S. 1989. A 100 MHz B-scan ultrasound backscatter microscope. Ultrason. Imag. 11, 95–105 10.1016/0161-7346(89)90002-3 (doi:10.1016/0161-7346(89)90002-3) - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
