Biomedical photoacoustic imaging
- PMID: 22866233
- PMCID: PMC3262268
- DOI: 10.1098/rsfs.2011.0028
Biomedical photoacoustic imaging
Abstract
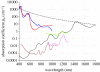
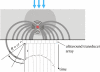
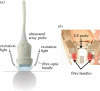
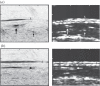
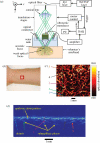
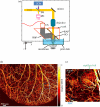
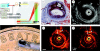
Photoacoustic (PA) imaging, also called optoacoustic imaging, is a new biomedical imaging modality based on the use of laser-generated ultrasound that has emerged over the last decade. It is a hybrid modality, combining the high-contrast and spectroscopic-based specificity of optical imaging with the high spatial resolution of ultrasound imaging. In essence, a PA image can be regarded as an ultrasound image in which the contrast depends not on the mechanical and elastic properties of the tissue, but its optical properties, specifically optical absorption. As a consequence, it offers greater specificity than conventional ultrasound imaging with the ability to detect haemoglobin, lipids, water and other light-absorbing chomophores, but with greater penetration depth than purely optical imaging modalities that rely on ballistic photons. As well as visualizing anatomical structures such as the microvasculature, it can also provide functional information in the form of blood oxygenation, blood flow and temperature. All of this can be achieved over a wide range of length scales from micrometres to centimetres with scalable spatial resolution. These attributes lend PA imaging to a wide variety of applications in clinical medicine, preclinical research and basic biology for studying cancer, cardiovascular disease, abnormalities of the microcirculation and other conditions. With the emergence of a variety of truly compelling in vivo images obtained by a number of groups around the world in the last 2-3 years, the technique has come of age and the promise of PA imaging is now beginning to be realized. Recent highlights include the demonstration of whole-body small-animal imaging, the first demonstrations of molecular imaging, the introduction of new microscopy modes and the first steps towards clinical breast imaging being taken as well as a myriad of in vivo preclinical imaging studies. In this article, the underlying physical principles of the technique, its practical implementation, and a range of clinical and preclinical applications are reviewed.
Keywords: biomedical; imaging; medical; photoacoustic; ultrasound.
Figures


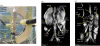





References
-
- Bell A. G. 1880. On the production and reproduction of sound by light. Am. J. Sci. 20, 305–324.
-
- Esenaliev R. O., Karabutov A. A., Tittel F. K., Fornage B. D., Thomsen S. L., Stelling C., Oraevsky A. A. 1997. Laser optoacoustic imaging for breast cancer diagnostics: limit of detection and comparison with x-ray and ultrasound imaging. Proc. SPIE 2979, 71–82.10.1117/12.280213 (10.1117/12.280213) - DOI - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
