Glutathione-S-transferase M1 regulation of diesel exhaust particle-induced pro-inflammatory mediator expression in normal human bronchial epithelial cells
- PMID: 22867088
- PMCID: PMC3480908
- DOI: 10.1186/1743-8977-9-31
Glutathione-S-transferase M1 regulation of diesel exhaust particle-induced pro-inflammatory mediator expression in normal human bronchial epithelial cells
Abstract
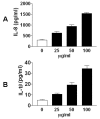
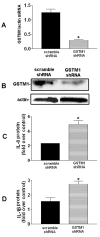
Background: Diesel exhaust particles (DEP) contribute substantially to ambient particulate matter (PM) air pollution in urban areas. Inhalation of PM has been associated with increased incidence of lung disease in susceptible populations. We have demonstrated that the glutathione S-transferase M1 (GSTM1) null genotype could aggravate DEP-induced airway inflammation in human subjects. Given the critical role airway epithelial cells play in the pathogenesis of airway inflammation, we established the GSTM1 deficiency condition in primary bronchial epithelial cells from human volunteers with GSTM1 sufficient genotype (GSTM1+) using GSTM1 shRNA to determine whether GSTM1 deficiency could exaggerate DEP-induced expression of interleukin-8 (IL-8) and IL-1β proteins. Furthermore, the mechanisms underlying GSTM1 regulation of DEP-induced IL-8 and IL-1β expression were also investigated.
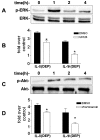
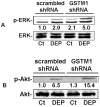
Methods: IL-8 and IL-1β protein levels were measured using enzyme-linked immunosorbent assay. GSTM1 deficiency in primary human bronchial epithelial cells was achieved using lentiviral GSTM1 shRNA particles and verified using real-time polymerase chain reaction and immunoblotting. Intracellular reactive oxygen species (ROS) production was evaluated using flow cytometry. Phosphorylation of protein kinases was detected using immunoblotting.
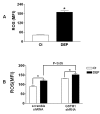
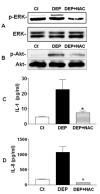
Results: Exposure of primary human bronchial epithelial cells (GSTM1+) to 25-100 μg/ml DEP for 24 h significantly increased IL-8 and IL-1β protein expression. Knockdown of GSTM1 in these cells further elevated DEP-induced IL-8 and IL-1β expression, implying that GSTM1 deficiency aggravated DEP-induced pro-inflammatory response. DEP stimulation induced the phosphorylation of extracellular signal-regulated kinase (ERK) and Akt, the downstream kinase of phosphoinositide 3-kinase (PI3K), in GSTM1+ bronchial epithelial cells. Pharmacological inhibition of ERK kinase and PI3K activity blocked DEP-induced IL-8 and IL-1β expression. DEP-induced ERK and Akt phosphorylation could be increased by GSTM1 knockdown. In addition, pretreatment of HBEC with the antioxidant N-acetyl cysteine significantly inhibited DEP-induced ERK and Akt phosphorylation, and subsequent IL-8 and IL-1β expression.
Conclusion: GSTM1 regulates DEP-induced IL-8 and IL-1β expression in primary human bronchial epithelial cells by modulation of ROS, ERK and Akt signaling.
Figures
References
-
- McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, Harrington R, Svartengren M, Han IK, Ohman-Strickland P, Chung KF, Zhang J. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. - DOI - PubMed
-
- Ris C. U.S. EPA health assessment for diesel engine exhaust: a review. Inhal Toxicol. 2007;19(Suppl 1):229–239. - PubMed
-
- Raphael GD, Metcalfe DD. Mediators of airway inflammation. Eur J Respir Dis Suppl. 1986;147:44–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

