Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes
- PMID: 22867114
- PMCID: PMC3444651
- DOI: 10.1021/tx3002719
Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes
Abstract
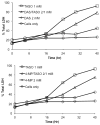
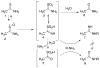
The hepatotoxicity of thioacetamide (TA) has been known since 1948. In rats, single doses cause centrolobular necrosis accompanied by increases in plasma transaminases and bilirubin. To elicit these effects, TA requires oxidative bioactivation, leading first to its S-oxide (TASO) and then to its chemically reactive S,S-dioxide (TASO(2)), which ultimately modifies amine-lipids and proteins. To generate a suite of liver proteins adducted by TA metabolites for proteomic analysis and to reduce the need for both animals and labeled compounds, we treated isolated hepatocytes directly with TA. Surprisingly, TA was not toxic at concentrations up to 50 mM for 40 h. On the other hand, TASO was highly toxic to isolated hepatocytes as indicated by LDH release, cellular morphology, and vital staining with Hoechst 33342/propidium iodide. TASO toxicity was partially blocked by the CYP2E1 inhibitors diallyl sulfide and 4-methylpyrazole and was strongly inhibited by TA. Significantly, we found that hepatocytes produce TA from TASO relatively efficiently by back-reduction. The covalent binding of [(14)C]-TASO is inhibited by unlabeled TA, which acts as a "cold-trap" for [(14)C]-TA and prevents its reoxidation to [(14)C]-TASO. This in turn increases the net consumption of [(14)C]-TASO despite the fact that its oxidation to TASO(2) is inhibited. The potent inhibition of TASO oxidation by TA, coupled with the back-reduction of TASO and its futile redox cycling with TA, may help explain phenomena previously interpreted as "saturation toxicokinetics" in the in vivo metabolism and toxicity of TA and TASO. The improved understanding of the metabolism and covalent binding of TA and TASO facilitates the use of hepatocytes to prepare protein adducts for target protein identification.
Figures








Similar articles
-
Protein targets of thioacetamide metabolites in rat hepatocytes.Chem Res Toxicol. 2013 Apr 15;26(4):564-74. doi: 10.1021/tx400001x. Epub 2013 Mar 20. Chem Res Toxicol. 2013. PMID: 23465048 Free PMC article.
-
Covalent modification of lipids and proteins in rat hepatocytes and in vitro by thioacetamide metabolites.Chem Res Toxicol. 2012 Sep 17;25(9):1868-77. doi: 10.1021/tx3001658. Epub 2012 Jun 15. Chem Res Toxicol. 2012. PMID: 22667464 Free PMC article.
-
Toxicokinetics and toxicity of thioacetamide sulfoxide: a metabolite of thioacetamide.Toxicology. 2007 Feb 12;230(2-3):105-16. doi: 10.1016/j.tox.2006.11.050. Epub 2006 Nov 17. Toxicology. 2007. PMID: 17187915
-
Saturation toxicokinetics of thioacetamide: role in initiation of liver injury.Drug Metab Dispos. 2005 Dec;33(12):1877-85. doi: 10.1124/dmd.105.005520. Epub 2005 Sep 23. Drug Metab Dispos. 2005. PMID: 16183780
-
Potentiation of thioacetamide liver injury in diabetic rats is due to induced CYP2E1.J Pharmacol Exp Ther. 2000 Aug;294(2):473-9. J Pharmacol Exp Ther. 2000. PMID: 10900221
Cited by
-
Oncogenic plasmid DNA and liver injury agent dictates liver cancer development in a mouse model.Clin Sci (Lond). 2024 Oct 2;138(19):1227-1248. doi: 10.1042/CS20240560. Clin Sci (Lond). 2024. PMID: 39254423 Free PMC article.
-
Protein targets of thioacetamide metabolites in rat hepatocytes.Chem Res Toxicol. 2013 Apr 15;26(4):564-74. doi: 10.1021/tx400001x. Epub 2013 Mar 20. Chem Res Toxicol. 2013. PMID: 23465048 Free PMC article.
-
Amelioration of hepatic function, oxidative stress, and histopathologic damages by Cassia fistula L. fraction in thioacetamide-induced liver toxicity.Environ Sci Pollut Res Int. 2019 Oct;26(29):29930-29945. doi: 10.1007/s11356-019-06158-y. Epub 2019 Aug 13. Environ Sci Pollut Res Int. 2019. PMID: 31407268
-
Protective effect of Moringa oleifera leaves ethanolic extract against thioacetamide-induced hepatotoxicity in rats via modulation of cellular antioxidant, apoptotic and inflammatory markers.Environ Sci Pollut Res Int. 2019 Nov;26(31):32488-32504. doi: 10.1007/s11356-019-06368-4. Epub 2019 Oct 15. Environ Sci Pollut Res Int. 2019. PMID: 31617137
-
Animal models of drug-induced liver injury.Biochim Biophys Acta Mol Basis Dis. 2019 May 1;1865(5):1031-1039. doi: 10.1016/j.bbadis.2018.08.037. Epub 2018 Sep 3. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31007174 Free PMC article. Review.
References
-
- Fitzhugh OG, Nelson AA. Liver tumors in rats fed thiourea or thioacetamide. Science. 1948;108:626–628. - PubMed
-
- Kuroda K, Terao K, Akao M. Inhibitory effect of fumaric acid on hepatocarcinogenesis by thioacetamide in rats. J Natl Cancer Inst. 1987;79:1047–1051. - PubMed
-
- Yeh C-N, Maitra A, Lee K-F, Jan Y-Y, Chen M-F. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: An animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631–636. - PubMed
-
- Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J Hepatol. 2002;36:488–493. - PubMed
-
- Okuyama H, Makamura H, Shimahara Y, Uyama N, Kwon YW, Yamaoka N, Yodoi J. Overexprssison of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J Hepatol. 2002;42:117–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

