OSCILLATOR: A system for analysis of diurnal leaf growth using infrared photography combined with wavelet transformation
- PMID: 22867627
- PMCID: PMC3489599
- DOI: 10.1186/1746-4811-8-29
OSCILLATOR: A system for analysis of diurnal leaf growth using infrared photography combined with wavelet transformation
Abstract
Background: Quantification of leaf movement is an important tool for characterising the effects of environmental signals and the circadian clock on plant development. Analysis of leaf movement is currently restricted by the attachment of sensors to the plant or dependent upon visible light for time-lapse photography. The study of leaf growth movement rhythms in mature plants under biological relevant conditions, e.g. diurnal light and dark conditions, is therefore problematic.
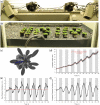
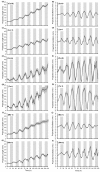
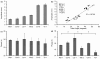
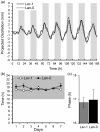
Results: Here we present OSCILLATOR, an affordable system for the analysis of rhythmic leaf growth movement in mature plants. The system contains three modules: (1) Infrared time-lapse imaging of growing mature plants (2) measurement of projected distances between leaf tip and plant apex (leaf tip tracking growth-curves) and (3) extraction of phase, period and amplitude of leaf growth oscillations using wavelet analysis. A proof-of-principle is provided by characterising parameters of rhythmic leaf growth movement of different Arabidopsis thaliana accessions as well as of Petunia hybrida and Solanum lycopersicum plants under diurnal conditions. The amplitude of leaf oscillations correlated to published data on leaf angles, while amplitude and leaf length did not correlate, suggesting a distinct leaf growth profile for each accession. Arabidopsis mutant accession Landsberg erecta displayed a late phase (timing of peak oscillation) compared to other accessions and this trait appears unrelated to the ERECTA locus.
Conclusions: OSCILLATOR is a low cost and easy to implement system that can accurately and reproducibly quantify rhythmic growth of mature plants for different species under diurnal light/dark cycling.
Figures
References
-
- Mancuso S, Shabala S, Moran N. Rhythms in Plants. Berlin Heidelberg: Springer; 2007. Rhythmic Leaf Movements: Physiological and Molecular Aspects; pp. 3–37.
-
- de Mairan J. Observation botanique. Hist Acad Roy Sci. 1729. pp. 35–36.
-
- Engelmann W, Simon K, Phen CJ. Leaf movement rhythms in Arabidopsis thaliana. Z Naturforsch. 1992;47:925–928.
LinkOut - more resources
Full Text Sources
Miscellaneous

