Histone deacetylase inhibitor suberoylanilide hydroxamic acid attenuates Toll-like receptor 4 signaling in lipopolysaccharide-stimulated mouse macrophages
- PMID: 22868051
- PMCID: PMC3496062
- DOI: 10.1016/j.jss.2012.07.023
Histone deacetylase inhibitor suberoylanilide hydroxamic acid attenuates Toll-like receptor 4 signaling in lipopolysaccharide-stimulated mouse macrophages
Abstract
Objective: We have previously demonstrated that pretreatment and posttreatment of animals with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, can improve survival in a mouse model of lipopolysaccharide (LPS)-induced severe shock. This study was designed to assess whether SAHA affects LPS/Toll-like receptor 4 signaling through acetylation of heat shock protein 90 (HSP90) and degradation of its client protein interleukin-1 receptor-associated kinase 1 (IRAK1).
Methods: RAW264.7 cells were exposed to LPS (1 μg/mL) for 2 h, followed by treatment with SAHA (10 μM) or geldanamycin (3 μM), an inhibitor of HSP90. Sham (no SAHA, no LPS) macrophages served as a control. The cells were harvested at different time points, and time zero served as the reference point.
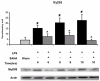
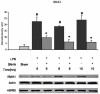
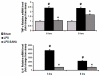
Results: LPS dramatically increased protein expression of myeloid differentiation factor 88 and IRAK1, and stimulated nuclear translocation of nuclear factor κB, leading to an increases of gene expression and protein production of tumor necrosis factor α and interleukin-6. Treatment with SAHA significantly attenuated these LPS-stimulated alterations. LPS or SAHA did not change the levels of HSP90 protein, but immunoprecipitation studies demonstrated that SAHA treatment enhanced acetylation of HSP90, and increased the dissociation of IRAK1, compared to the LPS control.
Conclusions: SAHA suppresses LPS/Toll-like receptor 4 signaling in LPS-stimulated macrophages through multiple potential mechanisms. It inhibits the function of HSP90 through hyperacetylation of the chaperone protein, which results in dissociation and degradation of the client protein IRAK1 and, at least in part, leads to a decrease in nuclear translocation of nuclear factor κB and attenuation of key proinflammatory cytokine expression.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

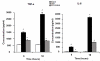

Comment in
-
Suberoylanilide hydroxamic acid (SAHA) as an agent to attenuate Toll-like receptor 4-induced septic shock.J Surg Res. 2013 Nov;185(1):e35-6. doi: 10.1016/j.jss.2012.09.004. Epub 2012 Sep 19. J Surg Res. 2013. PMID: 23073512 No abstract available.
Similar articles
-
Surviving lethal septic shock without fluid resuscitation in a rodent model.Surgery. 2010 Aug;148(2):246-54. doi: 10.1016/j.surg.2010.05.003. Epub 2010 Jun 19. Surgery. 2010. PMID: 20561658 Free PMC article.
-
Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents.Shock. 2009 Nov;32(5):517-23. doi: 10.1097/SHK.0b013e3181a44c79. Shock. 2009. PMID: 19295477
-
Gedunin Binds to Myeloid Differentiation Protein 2 and Impairs Lipopolysaccharide-Induced Toll-Like Receptor 4 Signaling in Macrophages.Mol Pharmacol. 2015 Nov;88(5):949-61. doi: 10.1124/mol.115.098970. Epub 2015 Sep 1. Mol Pharmacol. 2015. PMID: 26330549
-
Suberoylanilide hydroxamic acid (SAHA) as an agent to attenuate Toll-like receptor 4-induced septic shock.J Surg Res. 2013 Nov;185(1):e35-6. doi: 10.1016/j.jss.2012.09.004. Epub 2012 Sep 19. J Surg Res. 2013. PMID: 23073512 No abstract available.
-
Suberoylanilide Hydroxamic Acid (SAHA) Is a Driver Molecule of Neuroplasticity: Implication for Neurological Diseases.Biomolecules. 2023 Aug 24;13(9):1301. doi: 10.3390/biom13091301. Biomolecules. 2023. PMID: 37759701 Free PMC article. Review.
Cited by
-
Modified Suberoylanilide Hydroxamic Acid Reduced Drug-Associated Immune Cell Death and Organ Damage under Lipopolysaccharide Inflammatory Challenge.ACS Pharmacol Transl Sci. 2022 Oct 10;5(11):1128-1141. doi: 10.1021/acsptsci.2c00119. eCollection 2022 Nov 11. ACS Pharmacol Transl Sci. 2022. PMID: 36407956 Free PMC article.
-
Inhibition of histone deacetylase 6 improves long-term survival in a lethal septic model.J Trauma Acute Care Surg. 2015 Feb;78(2):378-85. doi: 10.1097/TA.0000000000000510. J Trauma Acute Care Surg. 2015. PMID: 25757125 Free PMC article.
-
Epigenetic control of macrophage polarization: implications for targeting tumor-associated macrophages.Oncotarget. 2018 Feb 21;9(29):20908-20927. doi: 10.18632/oncotarget.24556. eCollection 2018 Apr 17. Oncotarget. 2018. PMID: 29755698 Free PMC article. Review.
-
Inhibition of HDAC Enzymes Contributes to Differential Expression of Pro-Inflammatory Proteins in the TLR-4 Signaling Cascade.Int J Mol Sci. 2020 Nov 25;21(23):8943. doi: 10.3390/ijms21238943. Int J Mol Sci. 2020. PMID: 33255670 Free PMC article.
-
LPS-induced lipid alterations in microglia revealed by MALDI mass spectrometry-based cell fingerprinting in neuroinflammation studies.Sci Rep. 2022 Feb 21;12(1):2908. doi: 10.1038/s41598-022-06894-1. Sci Rep. 2022. PMID: 35190595 Free PMC article.
References
-
- Opal SM. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int J Med Microbiol. 2007;297:365–377. - PubMed
-
- Li Y, Liu B, Zhao H, Sailhamer EA, Fukudome EY, Zhang X, Kheirbek T, Finkelstein RA, Velmahos GC, deMoya M, Hales CA, Alam HB. Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents. Shock. 2009;32:517–523. - PubMed
-
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. - PubMed
-
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

