Innate immune responses in hepatitis C virus infection
- PMID: 22868377
- PMCID: PMC3732459
- DOI: 10.1007/s00281-012-0332-x
Innate immune responses in hepatitis C virus infection
Abstract
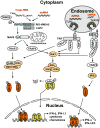
Hepatitis C virus (HCV) is a major causative agent of chronic hepatitis and hepatocellular carcinoma worldwide and thus poses a significant public health threat. A hallmark of HCV infection is the extraordinary ability of the virus to persist in a majority of infected people. Innate immune responses represent the front line of defense of the human body against HCV immediately after infection. They also play a crucial role in orchestrating subsequent HCV-specific adaptive immunity that is pivotal for viral clearance. Accumulating evidence suggests that the host has evolved multifaceted innate immune mechanisms to sense HCV infection and elicit defense responses, while HCV has developed elaborate strategies to circumvent many of these. Defining the interplay of HCV with host innate immunity reveals mechanistic insights into hepatitis C pathogenesis and informs approaches to therapy. In this review, we summarize recent advances in understanding innate immune responses to HCV infection, focusing on induction and effector mechanisms of the interferon antiviral response as well as the evasion strategies of HCV.
Figures


References
-
- Abe T, Kaname Y, Hamamoto I, Tsuda Y, Wen X, Taguwa S, Moriishi K, Takeuchi O, Kawai T, Kanto T, Hayashi N, Akira S, Matsuura Y. Hepatitis C virus nonstructural protein 5A modulates the toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J Virol. 2007;81:8953–8966. - PMC - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. - PubMed
-
- Aly HH, Watashi K, Hijikata M, Kaneko H, Takada Y, Egawa H, Uemoto S, Shimotohno K. Serum-derived hepatitis C virus infectivity in interferon regulatory factor-7-suppressed human primary hepatocytes. J Hepatol. 2007;46:26–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

