Stimulus-responsive nanopreparations for tumor targeting
- PMID: 22869005
- PMCID: PMC3521849
- DOI: 10.1039/c2ib20135f
Stimulus-responsive nanopreparations for tumor targeting
Abstract
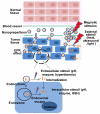
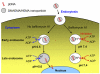
Nanopreparations such as liposomes, micelles, polymeric and inorganic nanoparticles, and small molecule/nucleic acid/protein conjugates have demonstrated various advantages over "naked" therapeutic molecules. These nanopreparations can be further engineered with functional moieties to improve their performance in terms of circulation longevity, targetability, enhanced intracellular penetration, carrier-mediated enhanced visualization, and stimuli-sensitivity. The idea of application of a stimulus-sensitive drug or imaging agent delivery system for tumor targeting is based on the significant abnormalities in the tumor microenvironment and its cells, such as an acidic pH, altered redox potential, up-regulated proteins and hyperthermia. These internal conditions as well as external stimuli, such as magnetic field, ultrasound and light, can be used to modify the behavior of the nanopreparations that control drug release, improve drug internalization, control the intracellular drug fate and even allow for certain physical interactions, resulting in an enhanced tumor targeting and antitumor effect. This article provides a critical view of current stimulus-sensitive drug delivery strategies and possible future directions in tumor targeting with primary focus on the combined use of stimulus-sensitivity with other strategies in the same nanopreparation, including multifunctional nanopreparations and theranostics.
Figures



References
-
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. - PubMed
-
- Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–30. DOI: 10.1021/nl102184c. - PMC - PubMed
- LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17:6437–47. DOI: 10.1158/1078-0432.CCR-11-0762. - PubMed
- Zhu L, Mahato RI. Lipid and polymeric carrier-mediated nucleic acid delivery. Expert Opin Drug Deliv. 2010;7:1209–26. DOI: 10.1517/17425247.2010.513969. - PMC - PubMed
-
- Wang FH, Kim DK, Yoshitake T, Johansson SM, Bjelke B, Muhammed M, Kehr J. Diffusion and clearance of superparamagnetic iron oxide nanoparticles infused into the rat striatum studied by MRI and histochemical techniques. Nanotechnology. 2011;22:015103. DOI: 10.1088/0957-4484/22/1/015103. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

