Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial
- PMID: 22869065
- PMCID: PMC3764914
- DOI: 10.1001/archneurol.2012.1970
Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial
Abstract
Background: Growth hormone–releasing hormone(GHRH), growth hormone, and insulin like growth factor 1 have potent effects on brain function, their levels decrease with advancing age, and they likely play a role in the pathogenesis of Alzheimer disease. Previously, we reported favorable cognitive effects of short-term GHRH administration in healthy older adults and provided preliminary evidence to suggest a similar benefit in adults with mild cognitive impairment (MCI).
Objective: To examine the effects of GHRH on cognitive function in healthy older adults and in adults with MCI.
Design: Randomized,double-blind,placebo-controlled trial.
Setting: Clinical Research Center, University of Washington School of Medicine in Seattle.
Participants: A total of 152 adults (66 with MCI) ranging in age from 55 to 87 years (mean age, 68 years); 137 adults (76 healthy participants and 61 participants with MCI) successfully completed the study.
Intervention: Participants self-administered daily subcutaneous injections of tesamorelin (Theratechnologies Inc),a stabilized analog of human GHRH (1 mg/d), or placebo 30 minutes before bedtime for 20 weeks. At baseline, at weeks 10 and 20 of treatment, and after a 10-week washout(week 30), blood samples were collected, and parallel versions of a cognitive battery were administered. Before and after the 20-week intervention, participants completed an oral glucose tolerance test and a dual-energy x-ray absorptiometry scan to measure body composition.
Main outcome measures: Primary cognitive outcomes were analyzed using analysis of variance and included 3 composites reflecting executive function, verbal memory, and visual memory. Executive function was assessed with Stroop Color-Word Interference,Task Switching, the Self-Ordered Pointing Test, and Word Fluency, verbal memory was assessed with Story Recall and the Hopkins Verbal Learning Test,and visual memory was assessed with the Visual-Spatial Learning Test and Delayed Match-to-Sample.
Results: The intent-to-treat analysis indicated a favorable effect of GHRH on cognition (P=.03), which was comparable in adults with MCI and healthy older adults.The completer analysis showed a similar pattern, with a more robust GHRH effect (P=.002). Subsequent analyses indicated a positive GHRH effect on executive function (P=.005) and a trend showing a similar treatment-related benefit in verbal memory(P=.08). Treatment with GHRH increased insulin like growth factor 1 levels by 117 %(P.001), which remained within the physiological range, and reduced percent body fat by 7.4%(P.001). Treatment with GHRH increased fasting insulin levels within the normal range by 35%in adults with MCI (P.001) but not in healthy adults. Adverse events were mild and were reported by 68%of GHRH treated adults and 36% of those who received placebo.
Conclusions: Twenty weeks of GHRH administration had favorable effects on cognition in both adults with MCI and healthy older adults. Longer-duration treatment trials are needed to further examine the therapeutic potential of GHRH administration on brain health during normal aging and “pathological aging.”
Trial registration: clinicaltrials.gov Identifier: NCT00257712
Figures

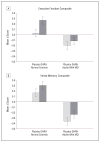
Comment in
-
Growth hormone-releasing hormone improves cognitive function in older adults: sleep on it.JAMA Neurol. 2013 Apr;70(4):529. doi: 10.1001/2013.jamaneurol.349. JAMA Neurol. 2013. PMID: 23568653 No abstract available.
-
Growth hormone-releasing hormone improves cognitive function in older adults: sleep on it--reply.JAMA Neurol. 2013 Apr;70(4):529-30. doi: 10.1001/jamaneurol.2013.2064. JAMA Neurol. 2013. PMID: 23568654 No abstract available.
References
-
- Thornton PL, Ingram RL, Sonntag WE. Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol A Biol Sci Med Sci. 2000;55(2):B106–B112. - PubMed
-
- Friedlander AL, Butterfield GE, Moynihan S, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86(4):1496–1503. - PubMed
-
- Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124(8):708–716. - PubMed
-
- Dhillon S. Tesamorelin: a review of its use in the management of HIV-associated lipodystrophy. Drugs. 2011;71(8):1071–1091. - PubMed
-
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4(2):195–212. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

