Estrogen facilitates spinal cord synaptic transmission via membrane-bound estrogen receptors: implications for pain hypersensitivity
- PMID: 22869379
- PMCID: PMC3460431
- DOI: 10.1074/jbc.M112.368142
Estrogen facilitates spinal cord synaptic transmission via membrane-bound estrogen receptors: implications for pain hypersensitivity
Abstract
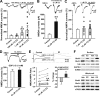
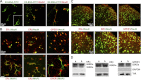
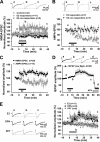
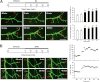
Recent evidence suggests that estrogen is synthesized in the spinal dorsal horn and plays a role in nociceptive processes. However, the cellular and molecular mechanisms underlying these effects remain unclear. Using electrophysiological, biochemical, and morphological techniques, we here demonstrate that 17β-estradiol (E2), a major form of estrogen, can directly modulate spinal cord synaptic transmission by 1) enhancing NMDA receptor-mediated synaptic transmission in dorsal horn neurons, 2) increasing glutamate release from primary afferent terminals, 3) increasing dendritic spine density in cultured spinal cord dorsal horn neurons, and 4) potentiating spinal cord long term potentiation (LTP) evoked by high frequency stimulation (HFS) of Lissauer's tract. Notably, E2-BSA, a ligand that acts only on membrane estrogen receptors, can mimic E2-induced facilitation of HFS-LTP, suggesting a nongenomic action of this neurosteroid. Consistently, cell surface biotinylation demonstrated that three types of ERs (ERα, ERβ, and GPER1) are localized on the plasma membrane of dorsal horn neurons. Furthermore, the ERα and ERβ antagonist ICI 182,780 completely abrogates the E2-induced facilitation of LTP. ERβ (but not ERα) activation can recapitulate E2-induced persistent increases in synaptic transmission (NMDA-dependent) and dendritic spine density, indicating a critical role of ERβ in spinal synaptic plasticity. E2 also increases the phosphorylation of ERK, PKA, and NR2B, and spinal HFS-LTP is prevented by blockade of PKA, ERK, or NR2B activation. Finally, HFS increases E2 release in spinal cord slices, which can be prevented by aromatase inhibitor androstatrienedione, suggesting activity-dependent local synthesis and release of endogenous E2.
Figures



References
-
- Maggiolini M., Picard D. (2010) The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J. Endocrinol. 204, 105–114 - PubMed
-
- Peng H. Y., Chen G. D., Tung K. C., Chien Y. W., Lai C. Y., Hsieh M. C., Chiu C. H., Lai C. H., Lee S. D., Lin T. B. (2009) Estrogen-dependent facilitation on spinal reflex potentiation involves the Cdk5/ERK1/2/NR2B cascade in anesthetized rats. Am. J. Physiol. Endocrinol. Metab. 297, E416–E426 - PubMed
-
- Liu N. J., Chakrabarti S., Schnell S., Wessendorf M., Gintzler A. R. (2011) Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal κ- and μ-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J. Neurosci. 31, 11836–11845 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

