A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability
- PMID: 22869557
- PMCID: PMC3496366
- DOI: 10.1158/1535-7163.MCT-12-0494
A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability
Abstract
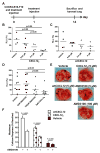
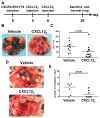
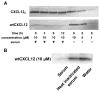
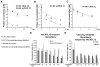
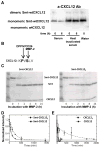
The CXC chemokine receptor-4 (CXCR4) plays a critical role in cancer by positively regulating cancer cell metastasis and survival. We previously showed that high concentrations of the CXCR4 ligand, wild-type CXCL12 (wtCXCL12), could inhibit colorectal cancer metastasis in vivo, and we have hypothesized that wtCXCL12 dimerizes at high concentration to become a potent antagonist of CXCR4. To address this hypothesis, we engineered a covalently locked, dimeric variant of CXCL12 (CXCL122). Herein, we show that CXCL122 can not only inhibit implantation of lung metastasis of CXCR4-B16-F10 melanoma cells more effectively than AMD3100, but that CXCL122 also blocks the growth of established pulmonary tumors. To identify a basis for the in vivo efficacy of CXCL122, we conducted Western blot analysis and ELISA analyses, which revealed that CXCL122 was stable for at least 12 hours in serum, whereas wtCXCL12 was quickly degraded. CXCL122 also maintained its antagonist properties in in vitro chemotaxis assays for up to 24 hours in serum, whereas wtCXCL12 was ineffective after 6 hours. Heat-inactivation of serum prolonged the stability and function of wtCXCL12 by more than 6 hours, suggesting enzymatic degradation as a possible mechanism for wtCXCL12 inactivation. In vitro analysis of amino-terminal cleavage by enzymes dipeptidylpeptidase IV (DPPIV/CD26) and matrix metalloproteinase-2 (MMP-2) resulted in 25-fold and 2-fold slower degradation rates, respectively, of CXCL122 compared with wtCXCL12. In summary, our results suggest CXCL122 possesses greater potential as an antimetastatic drug as compared with AMD3100 or wtCXCL12, potentially due to enhanced serum stability in the presence of N-terminal degrading enzymes.
©2012 AACR.
Conflict of interest statement
Disclosure: All authors have no conflict of interests.
Figures
Similar articles
-
Locked Dimerized CXCL12 Exerts Radiosensitizing Effects in Head and Neck Cancer.Head Neck. 2025 May;47(5):1379-1391. doi: 10.1002/hed.28048. Epub 2024 Dec 26. Head Neck. 2025. PMID: 39722591
-
AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis.Ann N Y Acad Sci. 2007 Jun;1106:1-19. doi: 10.1196/annals.1392.013. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360804
-
Inhibition of CXCR4 activity with AMD3100 decreases invasion of human colorectal cancer cells in vitro.World J Gastroenterol. 2008 Apr 21;14(15):2308-13. doi: 10.3748/wjg.14.2308. World J Gastroenterol. 2008. PMID: 18416455 Free PMC article.
-
The CXCR4/CXCL12 axis in cutaneous malignancies with an emphasis on melanoma.Histol Histopathol. 2014 Dec;29(12):1539-46. doi: 10.14670/HH-29.1539. Epub 2014 May 30. Histol Histopathol. 2014. PMID: 24879309 Review.
-
Potential of CXCR4 antagonists for the treatment of metastatic lung cancer.Expert Rev Anticancer Ther. 2011 Apr;11(4):621-30. doi: 10.1586/era.11.11. Expert Rev Anticancer Ther. 2011. PMID: 21504328 Review.
Cited by
-
The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization.Glycobiology. 2016 Mar;26(3):312-26. doi: 10.1093/glycob/cwv100. Epub 2015 Nov 17. Glycobiology. 2016. PMID: 26582609 Free PMC article.
-
A Requirement for Metamorphic Interconversion in the Antimicrobial Activity of Chemokine XCL1.Biochemistry. 2016 Jul 12;55(27):3784-93. doi: 10.1021/acs.biochem.6b00353. Epub 2016 Jun 28. Biochemistry. 2016. PMID: 27305837 Free PMC article.
-
Mesenchymal stem cell expression of stromal cell-derived factor-1β augments bone formation in a model of local regenerative therapy.J Orthop Res. 2015 Feb;33(2):174-84. doi: 10.1002/jor.22749. Epub 2014 Oct 28. J Orthop Res. 2015. PMID: 25351363 Free PMC article.
-
The Solution Structure of CCL28 Reveals Structural Lability that Does Not Constrain Antifungal Activity.J Mol Biol. 2018 Sep 14;430(18 Pt B):3266-3282. doi: 10.1016/j.jmb.2018.06.001. Epub 2018 Jun 15. J Mol Biol. 2018. PMID: 29913161 Free PMC article.
-
Regulating Chemokine-Receptor Interactions through the Site-Specific Bioorthogonal Conjugation of Photoresponsive DNA Strands.Bioconjug Chem. 2023 Nov 15;34(11):2089-2095. doi: 10.1021/acs.bioconjchem.3c00390. Epub 2023 Oct 19. Bioconjug Chem. 2023. PMID: 37856672 Free PMC article.
References
-
- Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25:357–71. - PubMed
-
- Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, et al. Expression of CXC chemokine receptor–4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–34. - PubMed
-
- Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

