Characterization of hepatitis C virus (HCV) quasispecies dynamics upon short-term dual therapy with the HCV NS5B nucleoside polymerase inhibitor mericitabine and the NS3/4 protease inhibitor danoprevir
- PMID: 22869576
- PMCID: PMC3486584
- DOI: 10.1128/AAC.01035-12
Characterization of hepatitis C virus (HCV) quasispecies dynamics upon short-term dual therapy with the HCV NS5B nucleoside polymerase inhibitor mericitabine and the NS3/4 protease inhibitor danoprevir
Abstract
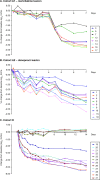
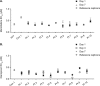
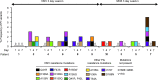
In the INFORM-1 study, 73 patients with chronic hepatitis C virus infection received mericitabine plus danoprevir for up to 13 days. Seventy-two patients experienced a continuous decline in HCV RNA levels during treatment, and of these patients, 14 had viral loads that remained >1,000 IU/ml by day 13 and 1 met the definition for viral breakthrough. In-depth NS5B and NS3/4A population and clonal sequencing studies and mericitabine and danoprevir drug susceptibility testing were performed to assess the variability and quasispecies dynamics before and upon monotherapy or dual therapy. Sequence analysis of the viral quasispecies indicated that the mericitabine resistance mutation S282T was not present at baseline, nor was it selected (even at a low level) during treatment. Protease inhibitor resistance mutations, either as predominant or as minority species, were detected in 18 patients at baseline. No enrichment of minority protease inhibitor-resistant variants present at baseline was observed during treatment; viral population samples were fully susceptible to mericitabine and/or danoprevir, despite the presence within their quasispecies of minority variants confirmed to have reduced susceptibility to danoprevir or other protease inhibitors. It was also observed that certain NS3 amino acid substitutions affected protease inhibitor drug susceptibility in a compound-specific manner and varied with the genetic context. In summary, the slower kinetics of viral load decline observed in some patients was not due to the selection of danoprevir or mericitabine resistance during treatment. Over 2 weeks' therapy, mericitabine suppressed the selection of danoprevir resistance, results that could differ upon longer treatment periods.
Figures
References
-
- Chayama K, et al. 2012. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology 55:742–748 - PubMed
-
- Forestier N, et al. 2011. Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN-191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study. J. Hepatol. 54:1130–1136 - PubMed
-
- Forestier N, et al. 2011. Antiviral activity of danoprevir (ITMN-191/RG7227) in combination with pegylated interferon alpha-2a and ribavirin in patients with hepatitis C. J. Infect. Dis. 204:601–608 - PubMed
-
- Forestier N, et al. 2007. Antiviral activity of telaprevir (VX-950) and peginterferon alfa-2a in patients with hepatitis C. Hepatology 46:640–648 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

