Nevirapine exposure with WHO pediatric weight band dosing: enhanced therapeutic concentrations predicted based on extensive international pharmacokinetic experience
- PMID: 22869579
- PMCID: PMC3457371
- DOI: 10.1128/AAC.00842-12
Nevirapine exposure with WHO pediatric weight band dosing: enhanced therapeutic concentrations predicted based on extensive international pharmacokinetic experience
Abstract
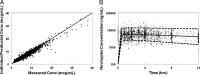
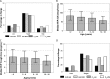
Nevirapine (NVP) is a nonnucleoside reverse transcriptase inhibitor (NNRTI) used worldwide as part of combination antiretroviral therapy in infants and children to treat HIV infection. Dosing based on either weight or body surface area has been approved by the U.S. Food and Drug Administration (FDA) but can be difficult to implement in resource-limited settings. The World Health Organization (WHO) has developed simplified weight band dosing for NVP, but it has not been critically evaluated. NVP pharmacokinetic data were combined from eight pediatric clinical trials (Pediatric AIDS Clinical Trials Group [PACTG] studies 245, 356, 366, 377, 403, 1056, and 1069 and Children with HIV in Africa Pharmacokinetics and Adherence of Simple Antiretroviral Regimens [CHAPAS]) representing subjects from multiple continents and across the pediatric age continuum. A population pharmacokinetic model was developed to characterize developmental changes in NVP disposition, identify potential sources of NVP pharmacokinetic variability, and assess various pediatric dosing strategies and their impact on NVP exposure. Age, CYP2B6 genotype, and ritonavir were independent predictors of oral NVP clearance. The Triomune fixed-dose tablet was an independent predictor of bioavailability compared to the liquid and other tablet formulations. Monte Carlo simulations of the final model were used to assess WHO weight band dosing recommendations. The final pharmacokinetic model indicated that WHO weight band dosing is likely to result in a percentage of children with NVP exposure within the target range similar to that obtained with FDA dosing. Weight band dosing of NVP proposed by the WHO has the potential to provide a simple and effective dosing strategy for resource limited settings.
Figures