Bodywide skipping of exons 45-55 in dystrophic mdx52 mice by systemic antisense delivery
- PMID: 22869723
- PMCID: PMC3427064
- DOI: 10.1073/pnas.1204638109
Bodywide skipping of exons 45-55 in dystrophic mdx52 mice by systemic antisense delivery
Abstract
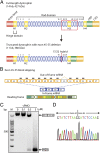
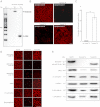
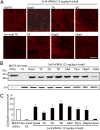
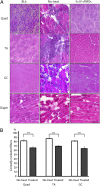
Duchenne muscular dystrophy (DMD), the commonest form of muscular dystrophy, is caused by lack of dystrophin. One of the most promising therapeutic approaches is antisense-mediated elimination of frame-disrupting mutations by exon skipping. However, this approach faces two major hurdles: limited applicability of each individual target exon and uncertain function and stability of each resulting truncated dystrophin. Skipping of exons 45-55 at the mutation hotspot of the DMD gene would address both issues. Theoretically it could rescue more than 60% of patients with deletion mutations. Moreover, spontaneous deletions of this specific region are associated with asymptomatic or exceptionally mild phenotypes. However, such multiple exon skipping of exons 45-55 has proved technically challenging. We have therefore designed antisense oligo (AO) morpholino mixtures to minimize self- or heteroduplex formation. These were tested as conjugates with cell-penetrating moieties (vivo-morpholinos). We have tested the feasibility of skipping exons 45-55 in H2K-mdx52 myotubes and in mdx52 mice, which lack exon 52. Encouragingly, with mixtures of 10 AOs, we demonstrated skipping of all 10 exons in vitro, in H2K-mdx52 myotubes and on intramuscular injection into mdx52 mice. Moreover, in mdx52 mice in vivo, systemic injections of 10 AOs induced extensive dystrophin expression at the subsarcolemma in skeletal muscles throughout the body, producing up to 15% of wild-type dystrophin protein levels, accompanied by improved muscle strength and histopathology without any detectable toxicity. This is a unique successful demonstration of effective rescue by exon 45-55 skipping in a dystrophin-deficient animal model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

