Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels
- PMID: 22869753
- PMCID: PMC3427074
- DOI: 10.1073/pnas.1205526109
Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels
Abstract
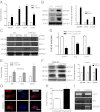
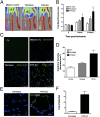
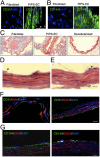
The generation of induced pluripotent stem (iPS) cells is an important tool for regenerative medicine. However, the main restriction is the risk of tumor development. In this study we found that during the early stages of somatic cell reprogramming toward a pluripotent state, specific gene expression patterns are altered. Therefore, we developed a method to generate partial-iPS (PiPS) cells by transferring four reprogramming factors (OCT4, SOX2, KLF4, and c-MYC) to human fibroblasts for 4 d. PiPS cells did not form tumors in vivo and clearly displayed the potential to differentiate into endothelial cells (ECs) in response to defined media and culture conditions. To clarify the mechanism of PiPS cell differentiation into ECs, SET translocation (myeloid leukemia-associated) (SET) similar protein (SETSIP) was indentified to be induced during somatic cell reprogramming. Importantly, when PiPS cells were treated with VEGF, SETSIP was translocated to the cell nucleus, directly bound to the VE-cadherin promoter, increasing vascular endothelial-cadherin (VE-cadherin) expression levels and EC differentiation. Functionally, PiPS-ECs improved neovascularization and blood flow recovery in a hindlimb ischemic model. Furthermore, PiPS-ECs displayed good attachment, stabilization, patency, and typical vascular structure when seeded on decellularized vessel scaffolds. These findings indicate that reprogramming of fibroblasts into ECs via SETSIP and VEGF has a potential clinical application.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Therapeutic transdifferentiation: a novel approach for vascular disease.Circ Res. 2013 Mar 1;112(5):748-50. doi: 10.1161/CIRCRESAHA.113.301053. Circ Res. 2013. PMID: 23449543 Free PMC article.
References
-
- Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. - PubMed
-
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Maherali N, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

