The chemokine receptors CXCR1 and CXCR2 couple to distinct G protein-coupled receptor kinases to mediate and regulate leukocyte functions
- PMID: 22869904
- PMCID: PMC3436986
- DOI: 10.4049/jimmunol.1201114
The chemokine receptors CXCR1 and CXCR2 couple to distinct G protein-coupled receptor kinases to mediate and regulate leukocyte functions
Abstract
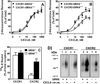
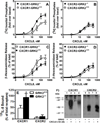
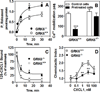
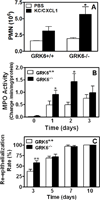
The chemokine receptors, CXCR1 and CXCR2, couple to Gαi to induce leukocyte recruitment and activation at sites of inflammation. Upon activation by CXCL8, these receptors become phosphorylated, desensitized, and internalized. In this study, we investigated the role of different G protein-coupled receptor kinases (GRKs) in CXCR1- and CXCR2-mediated cellular functions. To that end, short hairpin RNA was used to inhibit GRK2, 3, 5, and 6 in RBL-2H3 cells stably expressing CXCR1 or CXCR2, and CXCL8-mediated receptor activation and regulation were assessed. Inhibition of GRK2 and GRK6 increased CXCR1 and CXCR2 resistance to phosphorylation, desensitization, and internalization, respectively, and enhanced CXCL8-induced phosphoinositide hydrolysis and exocytosis in vitro. GRK2 depletion diminished CXCR1-induced ERK1/2 phosphorylation but had no effect on CXCR2-induced ERK1/2 phosphorylation. GRK6 depletion had no significant effect on CXCR1 function. However, peritoneal neutrophils from mice deficient in GRK6 (GRK6(-/-)) displayed an increase in CXCR2-mediated G protein activation but in vitro exhibited a decrease in chemotaxis, receptor desensitization, and internalization relative to wild-type (GRK6(+/+)) cells. In contrast, neutrophil recruitment in vivo in GRK6(-/-) mice was increased in response to delivery of CXCL1 through the air pouch model. In a wound-closure assay, GRK6(-/-) mice showed enhanced myeloperoxidase activity, suggesting enhanced neutrophil recruitment, and faster wound closure compared with GRK6(+/+) animals. Taken together, the results indicate that CXCR1 and CXCR2 couple to distinct GRK isoforms to mediate and regulate inflammatory responses. CXCR1 predominantly couples to GRK2, whereas CXCR2 interacts with GRK6 to negatively regulate receptor sensitization and trafficking, thus affecting cell signaling and angiogenesis.
Figures




References
-
- Baggiolini M. Reflections on chemokines. Immunol Rev. 2000;177:5–7. - PubMed
-
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. - PubMed
-
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. - PubMed
-
- Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem. 1998;273:23830–23836. - PubMed
-
- Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–2911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

