Soluble VEGF receptor-2 may be a predictive marker of anti-angiogenic therapy with clinically available safe agents
- PMID: 22870131
- PMCID: PMC3412527
- DOI: 10.3892/ol.2010.196
Soluble VEGF receptor-2 may be a predictive marker of anti-angiogenic therapy with clinically available safe agents
Abstract
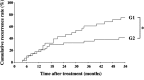
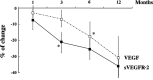
The identification of biomarkers of anti-angiogenic therapy that predict clinical benefit is of vital importance. We previously reported that a combination treatment with clinically available safe agents, specifically angiotensin-converting enzyme inhibitor (ACE-I) and vitamin K (VK), inhibited the cumulative recurrence of hepatocellular carcinoma (HCC) via suppression of the vascular endothelial growth factor (VEGF). The present study aimed to identify non-invasive biological markers that predict the clinically beneficial effect of this combination regimen. A combination of ACE-I (perindopril; 4 mg/day) and VK (menatetrenone; 45 mg/day) was administered for 54 months following curative therapy for HCC. The cumulative recurrence and several indices, which are reportedly considered as biological markers of anti-angiogenic therapies, were analyzed. The combined treatment of ACE-I and VK markedly inhibited the cumulative recurrence of HCC during the 54-month follow-up. The serum VEGF and soluble VEGF receptor (sVEGFR)-2 were significantly suppressed with this combination regimen, whereas sVEGFR-1 was not. In HCC patients without recurrence, a significant suppression of VEGF and sVEGFR-2 was achieved within 6 and 3 months after treatment, respectively. In conclusion, the combination treatment of ACE-I and VK is a potentially novel anti-angiogenic strategy for secondary chemoprevention against HCC since the two agents are widely used in clinical practice without serious side effects. Furthermore, sVEGFR-2 may become a useful clinical predictive marker of this combination treatment.
Figures

Similar articles
-
Combination of vitamin K2 and angiotensin-converting enzyme inhibitor ameliorates cumulative recurrence of hepatocellular carcinoma.J Hepatol. 2009 Aug;51(2):315-21. doi: 10.1016/j.jhep.2009.04.011. Epub 2009 May 15. J Hepatol. 2009. PMID: 19501932 Clinical Trial.
-
Combination of branched-chain amino acids and angiotensin-converting enzyme inhibitor suppresses the cumulative recurrence of hepatocellular carcinoma: a randomized control trial.Oncol Rep. 2011 Dec;26(6):1547-53. doi: 10.3892/or.2011.1433. Epub 2011 Aug 24. Oncol Rep. 2011. PMID: 21874260 Clinical Trial.
-
Amelioration of carcinogenesis and tumor growth in the rat liver by combination of vitamin K2 and angiotensin-converting enzyme inhibitor via anti-angiogenic activities.Oncol Rep. 2006 Jan;15(1):155-9. Oncol Rep. 2006. PMID: 16328049
-
Angiotensin-I-converting enzyme inhibitors may be an alternative anti-angiogenic strategy in the treatment of liver fibrosis and hepatocellular carcinoma. Possible role of vascular endothelial growth factor.Tumour Biol. 2002 Nov-Dec;23(6):348-56. doi: 10.1159/000069792. Tumour Biol. 2002. PMID: 12677092 Review.
-
Impact of renin-angiotensin system in hepatocellular carcinoma.Curr Cancer Drug Targets. 2011 May;11(4):431-41. doi: 10.2174/156800911795538084. Curr Cancer Drug Targets. 2011. PMID: 21395547 Review.
Cited by
-
Prognostic Factors for Overall Survival in Patients with HCV-Related HCC Undergoing Molecular Targeted Therapies: Beyond a Sustained Virological Response.Cancers (Basel). 2022 Oct 4;14(19):4850. doi: 10.3390/cancers14194850. Cancers (Basel). 2022. PMID: 36230773 Free PMC article.
-
Antitumor Effects and Mechanisms of Metabolic Syndrome Medications on Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2022 Dec 14;9:1279-1298. doi: 10.2147/JHC.S392051. eCollection 2022. J Hepatocell Carcinoma. 2022. PMID: 36545268 Free PMC article. Review.
-
Systematic review: Renin-angiotensin system inhibitors in chemoprevention of hepatocellular carcinoma.World J Gastroenterol. 2019 May 28;25(20):2524-2538. doi: 10.3748/wjg.v25.i20.2524. World J Gastroenterol. 2019. PMID: 31171895 Free PMC article.
References
-
- Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. - PubMed
-
- Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. - PubMed
-
- Guo RP, Zhong C, Shi M, et al. Clinical value of apoptosis and angiogenesis factors in estimating the prognosis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:547–555. - PubMed
-
- Iavarone M, Lampertico P, Iannuzzi F, et al. Increased expression of vascular endothelial growth factor in small hepatocellular carcinoma. J Viral Hepat. 2007;14:133–139. - PubMed
-
- Li CY, Shan S, Huang Q, et al. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–147. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
