Role of plastid transglutaminase in LHCII polyamination and thylakoid electron and proton flow
- PMID: 22870182
- PMCID: PMC3411467
- DOI: 10.1371/journal.pone.0041979
Role of plastid transglutaminase in LHCII polyamination and thylakoid electron and proton flow
Abstract
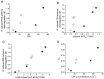
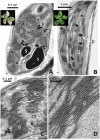
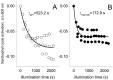
Transglutaminases function as biological glues in animal cells, plant cells and microbes. In energy producing organelles such as chloroplasts the presence of transglutaminases was recently confirmed. Furthermore, a plastidial transglutaminase has been cloned from maize and the first plants overexpressing tgz are available (Nicotiana tabacum TGZ OE). Our hypothesis is that the overexpression of plastidal transglutaminase will alter photosynthesis via increased polyamination of the antenna of photosystem II. We have used standard analytical tools to separate the antenna from photosystem II in wild type and modified plants, 6 specific antibodies against LHCbs to confirm their presence and sensitive HPLC method to quantify the polyamination level of these proteins. We report that bound spermidine and spermine were significantly increased (∼80%) in overexpressors. Moreover, we used recent advances in in vivo probing to study simultaneously the proton and electron circuit of thylakoids. Under physiological conditions overexpressors show a 3-fold higher sensitivity of the antenna down regulation loop (qE) to the elicitor (luminal protons) which is estimated as the ΔpH component of thylakoidal proton motive force. In addition, photosystem (hyper-PSIIα) with an exceptionally high antenna (large absorption cross section), accumulate in transglutaminase over expressers doubling the rate constant of light energy utilization (Kα) and promoting thylakoid membrane stacking. Polyamination of antenna proteins is a previously unrecognized mechanism for the modulation of the size (antenna absorption cross section) and sensitivity of photosystem II to down regulation. Future research will reveal which peptides and which residues of the antenna are responsible for such effects.
Conflict of interest statement
Figures









References
-
- Kramer DM, Cruz JA, Kanazawa A (2003) Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci 8: 27–32. - PubMed
-
- Pascal AA, Liu Z, Broess K, van Oort B, van Amerongen H, et al. (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436: 134–137. - PubMed

