Laropiprant attenuates EP3 and TP prostanoid receptor-mediated thrombus formation
- PMID: 22870195
- PMCID: PMC3411562
- DOI: 10.1371/journal.pone.0040222
Laropiprant attenuates EP3 and TP prostanoid receptor-mediated thrombus formation
Abstract
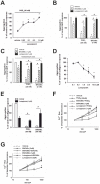
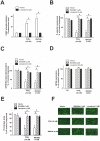
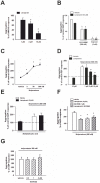
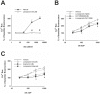
The use of the lipid lowering agent niacin is hampered by a frequent flush response which is largely mediated by prostaglandin (PG) D(2). Therefore, concomitant administration of the D-type prostanoid (DP) receptor antagonist laropiprant has been proposed to be a useful approach in preventing niacin-induced flush. However, antagonizing PGD(2), which is a potent inhibitor of platelet aggregation, might pose the risk of atherothrombotic events in cardiovascular disease. In fact, we found that in vitro treatment of platelets with laropiprant prevented the inhibitory effects of PGD(2) on platelet function, i.e. platelet aggregation, Ca(2+) flux, P-selectin expression, activation of glycoprotein IIb/IIIa and thrombus formation. In contrast, laropiprant did not prevent the inhibitory effects of acetylsalicylic acid or niacin on thrombus formation. At higher concentrations, laropiprant by itself attenuated platelet activation induced by thromboxane (TP) and E-type prostanoid (EP)-3 receptor stimulation, as demonstrated in assays of platelet aggregation, Ca(2+) flux, P-selectin expression, and activation of glycoprotein IIb/IIIa. Inhibition of platelet function exerted by EP4 or I-type prostanoid (IP) receptors was not affected by laropiprant. These in vitro data suggest that niacin/laropiprant for the treatment of dyslipidemias might have a beneficial profile with respect to platelet function and thrombotic events in vascular disease.
Conflict of interest statement
Figures
Similar articles
-
The prostaglandin E2 receptor EP4 is expressed by human platelets and potently inhibits platelet aggregation and thrombus formation.Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2416-23. doi: 10.1161/ATVBAHA.110.216374. Epub 2010 Nov 11. Arterioscler Thromb Vasc Biol. 2010. PMID: 21071691
-
The role of PGE(2) in human atherosclerotic plaque on platelet EP(3) and EP(4) receptor activation and platelet function in whole blood.J Thromb Thrombolysis. 2011 Aug;32(2):158-66. doi: 10.1007/s11239-011-0577-6. J Thromb Thrombolysis. 2011. PMID: 21424266
-
The role of prostanoid receptors in mediating the effects of PGE(2) on human platelet function.Platelets. 2010;21(5):329-42. doi: 10.3109/09537101003718065. Platelets. 2010. PMID: 20433310
-
Review of extended-release niacin/laropiprant fixed combination in the treatment of mixed dyslipidemia and primary hypercholesterolemia.Vasc Health Risk Manag. 2009;5:901-8. doi: 10.2147/vhrm.s4502. Epub 2009 Nov 16. Vasc Health Risk Manag. 2009. PMID: 20016845 Free PMC article. Review.
-
Mechanisms of flushing due to niacin and abolition of these effects.J Clin Hypertens (Greenwich). 2009 Nov;11(11):685-9. doi: 10.1111/j.1559-4572.2008.00050.x. J Clin Hypertens (Greenwich). 2009. PMID: 19878384 Free PMC article. Review.
Cited by
-
Platelet "first responders" in wound response, cancer, and metastasis.Cancer Metastasis Rev. 2017 Jun;36(2):199-213. doi: 10.1007/s10555-017-9682-0. Cancer Metastasis Rev. 2017. PMID: 28730545 Free PMC article. Review.
-
The EP1/EP3 receptor agonist 17-pt-PGE2 acts as an EP4 receptor agonist on endothelial barrier function and in a model of LPS-induced pulmonary inflammation.Vascul Pharmacol. 2016 Dec;87:180-189. doi: 10.1016/j.vph.2016.09.008. Epub 2016 Sep 21. Vascul Pharmacol. 2016. PMID: 27664754 Free PMC article.
-
Dietary niacin intake and mortality outcomes in hypertensive populations: analysis from NHANES 2003-2016.J Health Popul Nutr. 2025 Jun 18;44(1):206. doi: 10.1186/s41043-025-00976-2. J Health Popul Nutr. 2025. PMID: 40533845 Free PMC article.
-
Efficacy of Laropiprant in Minimizing Brain Injury Following Experimental Intracerebral Hemorrhage.Sci Rep. 2017 Aug 25;7(1):9489. doi: 10.1038/s41598-017-09994-5. Sci Rep. 2017. PMID: 28842638 Free PMC article.
-
Oxidized plasma albumin promotes platelet-endothelial crosstalk and endothelial tissue factor expression.Sci Rep. 2016 Feb 24;6:22104. doi: 10.1038/srep22104. Sci Rep. 2016. PMID: 26905525 Free PMC article.
References
-
- Armstrong RA (1996) Platelet prostanoid receptors. Pharmacol Ther 72: 171–91. - PubMed
-
- Svensson J, Hamberg M, Samuelsson B (1976) On the formation and effects of thromboxane A2 in human platelets. Acta Physiol Scand 98: 285–94. - PubMed
-
- Vane JR, Botting RM (1995) Pharmacodynamic profile of prostacyclin. Am J Cardiol 75: 3A–10A. - PubMed
-
- Bishop-Bailey D, Pepper JR, Haddad EB, Newton R, Larkin SW, et al. (1997) Induction of cyclooxygenase-2 in human saphenous vein and internal mammary artery. Arterioscler Thromb Vasc Biol 17: 1644–8. - PubMed
-
- Bishop-Bailey D, Pepper JR, Larkin SW, Mitchell JA (1998) Differential induction of cyclooxygenase-2 in human arterial and venous smooth muscle: role of endogenous prostanoids. Arterioscler Thromb Vasc Biol 18: 1655–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

