Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases
- PMID: 22870234
- PMCID: PMC3411714
- DOI: 10.1371/journal.pone.0041601
Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases
Abstract
Background: The population with multiple chronic conditions is growing. Prior studies indicate that patients with comorbidities are frequently excluded from trials but do not address whether information is available in trials to draw conclusions about treatment effects for these patients.
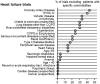
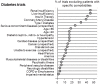
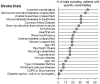
Methods and findings: We conducted a literature survey of trials from 11 Cochrane Reviews for four chronic diseases (diabetes, heart failure, chronic obstructive pulmonary disease, and stroke). The Cochrane Reviews systematically identified and summarized trials on the effectiveness of diuretics, metformin, anticoagulants, longacting beta-agonists alone or in combination with inhaled corticosteroids, lipid lowering agents, exercise and diet. Eligible studies were reports of trials included in the Cochrane reviews and additional papers that described the methods of these trials. We assessed the exclusion and inclusion of people with comorbidities, the reporting of comorbidities, and whether comorbidities were considered as potential modifiers of treatment effects. Overall, the replicability of both the inclusion criteria (mean [standard deviation (SD)]: 6.0 (2.1), range (min-max): 1-9.5) and exclusion criteria (mean(SD): 5.3 (2.1), range: 1-9.5) was only moderate. Trials excluded patients with many common comorbidities. The proportion of exclusions for comorbidities ranged from 0-42 percent for heart failure, 0-55 percent for COPD, 0-44 percent for diabetes, and 0-39 percent for stroke. Seventy of the 161 trials (43.5%) described the prevalence of any comorbidity among participants with the index disease. The reporting of comorbidities in trials was very limited, in terms of reporting an operational definition and method of ascertainment for the presence of comorbidity and treatments for the comorbidity. It was even less common that the trials assessed whether comorbidities were potential modifiers of treatment effects.
Conclusions: Comorbidities receive little attention in chronic disease trials. Given the public health importance of people with multiple chronic conditions, trials should better report on comorbidities and assess the effect comorbidities have on treatment outcomes.
Conflict of interest statement
Figures

References
-
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, et al. (2005) Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance JAMA. 294(6): 716–24. - PubMed
-
- US Department of Health and Human Services: Multiple Chronic Conditions – A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions.
-
- Parekh AK, Barton MB (2010) The challenge of multiple comorbidity for the US health care system. JAMA 303(13): 1303–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

