A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults
- PMID: 22870265
- PMCID: PMC3411704
- DOI: 10.1371/journal.pone.0041936
A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults
Abstract
Background: We conducted a phase I, randomized, double-blind, placebo-controlled trial to assess the safety and immunogenicity of escalating doses of two recombinant replication defective adenovirus serotype 35 (Ad35) vectors containing gag, reverse transcriptase, integrase and nef (Ad35-GRIN) and env (Ad35-ENV), both derived from HIV-1 subtype A isolates. The trial enrolled 56 healthy HIV-uninfected adults.
Methods: Ad35-GRIN/ENV (Ad35-GRIN and Ad35-ENV mixed in the same vial in equal proportions) or Ad35-GRIN was administered intramuscularly at 0 and 6 months. Participants were randomized to receive either vaccine or placebo (10/4 per group, respectively) within one of four dosage groups: Ad35-GRIN/ENV 2×10(9) (A), 2×10(10) (B), 2×10(11) (C), or Ad35-GRIN 1×10(10) (D) viral particles.
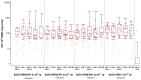
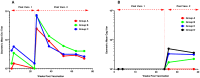
Results: No vaccine-related serious adverse event was reported. Reactogenicity events reported were dose-dependent, mostly mild or moderate, some severe in Group C volunteers, all transient and resolving spontaneously. IFN-γ ELISPOT responses to any vaccine antigen were detected in 50, 56, 70 and 90% after the first vaccination, and in 75, 100, 88 and 86% of Groups A-D vaccine recipients after the second vaccination, respectively. The median spot forming cells (SFC) per 10(6) PBMC to any antigen was 78-139 across Groups A-C and 158-174 in Group D, after each of the vaccinations with a maximum of 2991 SFC. Four to five HIV proteins were commonly recognized across all the groups and over multiple timepoints. CD4+ and CD8+ T-cell responses were polyfunctional. Env antibodies were detected in all Group A-C vaccinees and Gag antibodies in most vaccinees after the second immunization. Ad35 neutralizing titers remained low after the second vaccination.
Conclusion/significance: Ad35-GRIN/ENV reactogenicity was dose-related. HIV-specific cellular and humoral responses were seen in the majority of volunteers immunized with Ad35-GRIN/ENV or Ad35-GRIN and increased after the second vaccination. T-cell responses were broad and polyfunctional.
Trial registration: ClinicalTrials.gov NCT00851383.
Conflict of interest statement
Figures

Similar articles
-
A Phase I Double Blind, Placebo-Controlled, Randomized Study of the Safety and Immunogenicity of an Adjuvanted HIV-1 Gag-Pol-Nef Fusion Protein and Adenovirus 35 Gag-RT-Int-Nef Vaccine in Healthy HIV-Uninfected African Adults.PLoS One. 2015 May 11;10(5):e0125954. doi: 10.1371/journal.pone.0125954. eCollection 2015. PLoS One. 2015. PMID: 25961283 Free PMC article. Clinical Trial.
-
First-in-Human Evaluation of the Safety and Immunogenicity of an Intranasally Administered Replication-Competent Sendai Virus-Vectored HIV Type 1 Gag Vaccine: Induction of Potent T-Cell or Antibody Responses in Prime-Boost Regimens.J Infect Dis. 2017 Jan 1;215(1):95-104. doi: 10.1093/infdis/jiw500. Epub 2016 Oct 17. J Infect Dis. 2017. PMID: 28077588 Free PMC article. Clinical Trial.
-
Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector.J Infect Dis. 2006 Dec 15;194(12):1638-49. doi: 10.1086/509258. Epub 2006 Nov 8. J Infect Dis. 2006. PMID: 17109335 Free PMC article. Clinical Trial.
-
Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine.Curr Opin HIV AIDS. 2010 Sep;5(5):357-61. doi: 10.1097/COH.0b013e32833d2d2b. Curr Opin HIV AIDS. 2010. PMID: 20978374 Free PMC article. Review.
-
Vaccine applications of flow cytometry.Methods. 2012 Jul;57(3):383-91. doi: 10.1016/j.ymeth.2012.01.001. Epub 2012 Jan 10. Methods. 2012. PMID: 22251671 Free PMC article. Review.
Cited by
-
Characterization of a live-attenuated HCMV-based vaccine platform.Sci Rep. 2019 Dec 17;9(1):19236. doi: 10.1038/s41598-019-55508-w. Sci Rep. 2019. PMID: 31848362 Free PMC article.
-
The influence of delivery vectors on HIV vaccine efficacy.Front Microbiol. 2014 Aug 22;5:439. doi: 10.3389/fmicb.2014.00439. eCollection 2014. Front Microbiol. 2014. PMID: 25202303 Free PMC article. Review.
-
2022 World AIDS day: Past achievements and future optimism.New Microbes New Infect. 2022 Dec 17;51:101067. doi: 10.1016/j.nmni.2022.101067. eCollection 2023 Jan. New Microbes New Infect. 2022. PMID: 36593884 Free PMC article. No abstract available.
-
Measuring Cellular Immunity to Influenza: Methods of Detection, Applications and Challenges.Vaccines (Basel). 2015 Apr 14;3(2):293-319. doi: 10.3390/vaccines3020293. Vaccines (Basel). 2015. PMID: 26343189 Free PMC article. Review.
-
New developments in an old strategy: heterologous vector primes and envelope protein boosts in HIV vaccine design.Expert Rev Vaccines. 2016 Aug;15(8):1015-27. doi: 10.1586/14760584.2016.1158108. Epub 2016 Mar 16. Expert Rev Vaccines. 2016. PMID: 26910195 Free PMC article. Review.
References
-
- UNAIDS (2011) Global HIV/AIDS Response. Epidemic update and health sector progress towards Universal Access. Progress Report 2011.
-
- Fauci AS, Johnston MI, Dieffenbach CW, Burton DR, Hammer SM, et al. (2008) HIV vaccine research: the way forward. Science 321: 530–532. - PubMed
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, et al. (2005) Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 191: 654–665. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

