The proteolytic activity of separase in BCR-ABL-positive cells is increased by imatinib
- PMID: 22870341
- PMCID: PMC3411713
- DOI: 10.1371/journal.pone.0042863
The proteolytic activity of separase in BCR-ABL-positive cells is increased by imatinib
Abstract
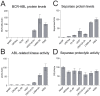
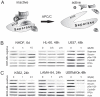
Separase, an endopeptidase required for the separation of sister-chromatides in mitotic anaphase, triggers centriole disengagement during centrosome duplication. In cancer, separase is frequently overexpressed, pointing to a functional role as an aneuploidy promoter associated with centrosomal amplification and genomic instability. Recently, we have shown that centrosomal amplification and subsequent chromosomal aberrations are a hallmark of chronic myeloid leukemia (CML), increasing from chronic phase (CP) toward blast crisis (BC). Moreover, a functional linkage of p210BCR-ABL tyrosine kinase activity with centrosomal amplification and clonal evolution has been established in long-term cell culture experiments. Unexpectedly, therapeutic doses of imatinib (IM) did not counteract; instead induced similar centrosomal alterations in vitro. We investigated the influence of IM and p210BCR-ABL on Separase as a potential driver of centrosomal amplification in CML. Short-term cell cultures of p210BCR-ABL-negative (NHDF, UROtsa, HL-60, U937), positive (K562, LAMA-84) and inducible (U937p210BCR-ABL/c6 (Tet-ON)) human cell lines were treated with therapeutic doses of IM and analyzed by qRT-PCR, Western blot analysis and quantitative Separase activity assays. Decreased Separase protein levels were observed in all cells treated with IM in a dose dependent manner. Accordingly, in all p210BCR-ABL-negative cell lines, decreased proteolytic activity of Separase was found. In contrast, p210BCR-ABL-positive cells showed increased Separase proteolytic activity. This activation of Separase was consistent with changes in the expression levels of Separase regulators (Separase phosphorylation at serine residue 1126, Securin, CyclinB1 and PP2A). Our data suggest that regulation of Separase in IM-treated BCR-ABL-positive cells occurs on both the protein expression and the proteolytic activity levels. Activation of Separase proteolytic activity exclusively in p210BCR-ABL-positive cells during IM treatment may act as a driving force for centrosomal amplification, contributing to genomic instability, clonal evolution and resistance in CML.
Conflict of interest statement
Figures



References
-
- Sawyers CL (1999) Chronic myeloid leukemia. N Engl J Med 340: 1330–1340. - PubMed
-
- Calabretta B, Perrotti D (2004) The biology of CML blast crisis. Blood 103: 4010–4022. - PubMed
-
- Kumari A, Brendel C, Hochhaus A, Neubauer A, Burchert A (2011) Low BCR-ABL expression levels in hematopoietic precursor cells enable persistence of chronic myeloid leukemia under imatinib. Blood 119: 530–539. - PubMed
-
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, et al. (2006) Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355: 2408–2417. - PubMed
-
- Hochhaus A, Schenk T, Erben P, Ernst T, La Rosee P, et al. (2009) Cause and management of therapy resistance. Best Pract Res Clin Haematol 22: 367–379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

