Systems analysis of transcription factor activities in environments with stable and dynamic oxygen concentrations
- PMID: 22870390
- PMCID: PMC3411108
- DOI: 10.1098/rsob.120091
Systems analysis of transcription factor activities in environments with stable and dynamic oxygen concentrations
Abstract
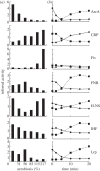
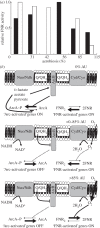
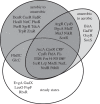
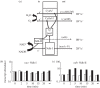
Understanding gene regulation requires knowledge of changes in transcription factor (TF) activities. Simultaneous direct measurement of numerous TF activities is currently impossible. Nevertheless, statistical approaches to infer TF activities have yielded non-trivial and verifiable predictions for individual TFs. Here, global statistical modelling identifies changes in TF activities from transcript profiles of Escherichia coli growing in stable (fixed oxygen availabilities) and dynamic (changing oxygen availability) environments. A core oxygen-responsive TF network, supplemented by additional TFs acting under specific conditions, was identified. The activities of the cytoplasmic oxygen-responsive TF, FNR, and the membrane-bound terminal oxidases implied that, even on the scale of the bacterial cell, spatial effects significantly influence oxygen-sensing. Several transcripts exhibited asymmetrical patterns of abundance in aerobic to anaerobic and anaerobic to aerobic transitions. One of these transcripts, ndh, encodes a major component of the aerobic respiratory chain and is regulated by oxygen-responsive TFs ArcA and FNR. Kinetic modelling indicated that ArcA and FNR behaviour could not explain the ndh transcript profile, leading to the identification of another TF, PdhR, as the source of the asymmetry. Thus, this approach illustrates how systematic examination of regulatory responses in stable and dynamic environments yields new mechanistic insights into adaptive processes.
Keywords: Escherichia coli; mathematical modelling; oxygen-sensing; systems biology; transcript profiling.
Figures

References
-
- Guest JR, Green J, Irvine AS, Spiro S. 1996. The FNR modulon and FNR-regulated gene expression. In Regulation of gene expression in Escherichia coli (eds Lin ECC, Lynch AS.), pp. 317–342 Austin, TX: R. G. Landes, Co
-
- Gennis RB, Stewart V. 1996. Respiration. In Escherichia coli and Salmonella cellular and molecular biology (ed. Neidhardt FC.), pp. 217–261, 2nd edn Washington, DC: ASM Press
-
- Bock A, Sawers G. 1996. Fermentation. In Escherichia coli and Salmonella cellular and molecular biology (ed. Neidhardt FC.), pp. 262–282, 2nd edn Washington, DC: ASM Press
-
- Rolfe MD, Ter Beek A, Graham AI, Trotter EW, Asif HMS, Sanguinetti G, Teixeeira de Mattos J, Poole RK, Green J. 2011. Transcript profiling and inference of Escherichia coli K-12 ArcA activity across the range of physiologically relevant oxygen concentrations. J. Biol. Chem. 286, 10 147–10 15410.1074/jbcM110211144 (doi:10.1074/jbcM110211144) - DOI - DOI - PMC - PubMed
-
- Partridge JD, Scott C, Tang Y, Poole RK, Green J. 2006. Escherichia coli transcriptome dynamics during transition from anaerobic to aerobic conditions. J. Biol. Chem. 281, 27 806–27 81510.107/jbcM603450200 (doi:10.107/jbcM603450200) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

