The future of warfarin pharmacogenetics in under-represented minority groups
- PMID: 22871196
- PMCID: PMC3463230
- DOI: 10.2217/fca.12.31
The future of warfarin pharmacogenetics in under-represented minority groups
Abstract
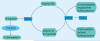
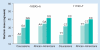
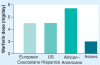
Genotype-based dosing recommendations are provided in the US FDA-approved warfarin labeling. However, data that informed these recommendations were from predominately Caucasian populations. Studies show that variants contributing to warfarin dose requirements in Caucasians provide similar contributions to dose requirements in US Hispanics, but significantly lesser contributions in African-Americans. Further data demonstrate that variants occurring commonly in individuals of African ancestry, but rarely in other racial groups, significantly influence dose requirements in African-Americans. These data suggest that it is important to consider variants specific for African-Americans when implementing genotype-guided warfarin dosing in this population.
Figures

References
-
- Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with a trial fibrillation. Circulation. 2007;115(21):2689–2696. - PubMed
-
- Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N. Engl. J. Med. 2003;349(11):1019–1026. - PubMed
-
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011;365(21):2002–2012. - PubMed
-
- Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition). Chest. 2008;133(Suppl. 6):S160–S198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
