Nitric oxide synthases in heart failure
- PMID: 22871241
- PMCID: PMC3567782
- DOI: 10.1089/ars.2012.4824
Nitric oxide synthases in heart failure
Abstract
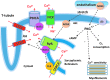
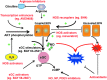
Significance: The regulation of myocardial function by constitutive nitric oxide synthases (NOS) is important for the maintenance of myocardial Ca(2+) homeostasis, relaxation and distensibility, and protection from arrhythmia and abnormal stress stimuli. However, sustained insults such as diabetes, hypertension, hemodynamic overload, and atrial fibrillation lead to dysfunctional NOS activity with superoxide produced instead of NO and worse pathophysiology.
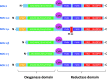
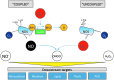
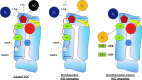
Recent advances: Major strides in understanding the role of normal and abnormal constitutive NOS in the heart have revealed molecular targets by which NO modulates myocyte function and morphology, the role and nature of post-translational modifications of NOS, and factors controlling nitroso-redox balance. Localized and differential signaling from NOS1 (neuronal) versus NOS3 (endothelial) isoforms are being identified, as are methods to restore NOS function in heart disease.
Critical issues: Abnormal NOS signaling plays a key role in many cardiac disorders, while targeted modulation may potentially reverse this pathogenic source of oxidative stress.
Future directions: Improvements in the clinical translation of potent modulators of NOS function/dysfunction may ultimately provide a powerful new treatment for many hearts diseases that are fueled by nitroso-redox imbalance.
Figures
References
-
- Adachi T. Modulation of vascular sarco/endoplasmic reticulum calcium ATPase in cardiovascular pathophysiology. Adv Pharmacol. 2010;59:165–195. - PubMed
-
- Aicher A. Heeschen C. Mildner-Rihm C. Urbich C. Ihling C. Technau-Ihling K. Zeiher AM. Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

