Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies
- PMID: 22871339
- PMCID: PMC3469619
- DOI: 10.1152/ajpendo.00279.2012
Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies
Abstract
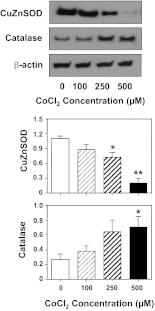
Vitamin D insufficiency/deficiency during pregnancy has been linked to increased risk of preeclampsia. Placenta dysfunction plays an important role in the pathogenesis of this pregnancy disorder. In this study, we tested the hypothesis that disturbed vitamin D metabolism takes place in preeclamptic placentas. Protein expressions of vitamin D binding protein (VDBP), 25-hydroxylase (CYP2R1), 1α-hydroxylase (CYP27B1), 24-hydroxylase (CYP24A1), and vitamin D receptor (VDR) were examined in placentas from normotensive and preeclamptic pregnancies. By immunostaining we found that in normal placenta VDBP, CYP24A1, and VDR expressions are localized mainly in trophoblasts, whereas CYP2R1 and CYP27B1 expressions are localized mainly in villous core fetal vessel endothelium. Protein expressions of CYP2R1 and VDR are reduced, but CYP27B1 and CYP24A1 expressions are elevated, in preeclamptic compared with normotensive placentas. Because increased oxidative stress is an underlying pathophysiology in placental trophoblasts in preeclampsia, we further determined whether oxidative stress contributes to altered vitamin D metabolic system in placental trophoblasts. Trophoblasts isolated from normal-term placentas were treated with hypoxic-inducing agent CoCl(2), and protein expressions of VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR were determined. We found that hypoxia-induced downregulation of VDBP, CYP2R1, and VDR and upregulation of CYP27B1 and CYP24A1 expressions were consistent with that seen in preeclamptic placentas. CuZnSOD expression was also downregulated in trophoblasts treated with CoCl(2). These results provide direct evidence of disrupted vitamin D metabolic homeostasis in the preeclamptic placenta and suggest that increased oxidative stress could be a causative factor of altered vitamin D metabolism in preeclamptic placentas.
Figures


Similar articles
-
Altered decidual and placental catabolism of vitamin D may contribute to the aetiology of spontaneous miscarriage.Placenta. 2020 Mar;92:1-8. doi: 10.1016/j.placenta.2020.01.013. Epub 2020 Jan 26. Placenta. 2020. PMID: 32056782
-
Reprint of "Vitamin D deficiency in pregnant women impairs regulatory T cell function".J Steroid Biochem Mol Biol. 2015 Apr;148:194-201. doi: 10.1016/j.jsbmb.2015.01.026. Epub 2015 Jan 31. J Steroid Biochem Mol Biol. 2015. PMID: 25644204
-
Vitamin D Metabolic Pathway Genes Polymorphisms and Their Methylation Levels in Association With Rheumatoid Arthritis.Front Immunol. 2021 Dec 2;12:731565. doi: 10.3389/fimmu.2021.731565. eCollection 2021. Front Immunol. 2021. PMID: 34925313 Free PMC article.
-
Metabolism of vitamin D3 by cytochromes P450.Front Biosci. 2005 Jan 1;10:119-34. doi: 10.2741/1514. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15574355 Review.
-
[Recent studies on vitamin D metabolizing enzymes].Clin Calcium. 2006 Jul;16(7):1129-35. Clin Calcium. 2006. PMID: 16816472 Review. Japanese.
Cited by
-
[Prevalence of vitamin D deficiency and associated factors in women and newborns in the immediate postpartum period].Rev Paul Pediatr. 2015 Jul-Sep;33(3):287-94. doi: 10.1016/j.rpped.2015.01.006. Epub 2015 Jun 9. Rev Paul Pediatr. 2015. PMID: 26100593 Free PMC article.
-
Clinical Usefulness of Bioavailable Vitamin D and Impact of GC Genotyping on the Determination of Bioavailable Vitamin D in a Korean Population.Int J Endocrinol. 2019 Jan 13;2019:9120467. doi: 10.1155/2019/9120467. eCollection 2019. Int J Endocrinol. 2019. PMID: 30774661 Free PMC article.
-
Could Vitamin D Be Effective in Prevention of Preeclampsia?Nutrients. 2021 Oct 28;13(11):3854. doi: 10.3390/nu13113854. Nutrients. 2021. PMID: 34836111 Free PMC article. Review.
-
Placental genetic variations in vitamin D metabolism and birthweight.Placenta. 2017 Feb;50:78-83. doi: 10.1016/j.placenta.2016.12.028. Epub 2016 Dec 27. Placenta. 2017. PMID: 28161065 Free PMC article.
-
Physiology of Vitamin D-Focusing on Disease Prevention.Nutrients. 2024 May 29;16(11):1666. doi: 10.3390/nu16111666. Nutrients. 2024. PMID: 38892599 Free PMC article. Review.
References
-
- Ainy E, Ghazi AA, Azizi F. Changes in calcium, 25(OH) vitamin D3 and other biochemical factors during pregnancy. J Endocrinol Invest 29: 303–307, 2006 - PubMed
-
- Awad AB, Alappat L, Valerio M. Vitamin D and metabolic syndrome risk factors: evidence and mechanisms. Crit Rev Food Sci Nutr 52: 103–112, 2012 - PubMed
-
- Bikle DD. Vitamin D regulation of immune function. Vitam Horm 86: 1–21, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

