Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration
- PMID: 22871377
- PMCID: PMC3557444
- DOI: 10.1089/ten.TEC.2012.0221
Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration
Abstract
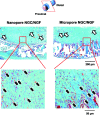
Polycaprolactone (PCL)/Pluronic F127 nerve guide conduits (NGCs) with different surface pore structures (nano-porous inner surface vs. micro-porous inner surface) but similar physical and chemical properties were fabricated by rolling the opposite side of asymmetrically porous PCL/F127 membranes. The effect of the pore structure on peripheral nerve regeneration through the NGCs was investigated using a sciatic nerve defect model of rats. The nerve fibers and tissues were shown to have regenerated along the longitudinal direction through the NGC with a nano-porous inner surface (Nanopore NGC), while they grew toward the porous wall of the NGC with a micro-porous inner surface (Micropore NGC) and, thus, their growth was restricted when compared with the Nanopore NGC, as investigated by immunohistochemical evaluations (by fluorescence microscopy with anti-neurofilament staining and Hoechst staining for growth pattern of nerve fibers), histological evaluations (by light microscopy with Meyer's modified trichrome staining and Toluidine blue staining and transmission electron microscopy for the regeneration of axon and myelin sheath), and FluoroGold retrograde tracing (for reconnection between proximal and distal stumps). The effect of nerve growth factor (NGF) immobilized on the pore surfaces of the NGCs on nerve regeneration was not so significant when compared with NGCs not containing immobilized NGF. The NGC system with different surface pore structures but the same chemical/physical properties seems to be a good tool that is used for elucidating the surface pore effect of NGCs on nerve regeneration.
Figures










References
-
- Zhang N. Yan H. Wen X. Tissue-engineering approaches for axonal guidance. Brain Res Brain Res Rev. 2005;49:48. - PubMed
-
- Seckel B.R. Enhancement of peripheral nerve regeneration. Muscle Nerve. 1990;13:785. - PubMed
-
- Schlosshauer B. Müller E. Schröder B. Planck H. Müller H.W. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 2003;963:321. - PubMed
-
- Glück T. Ueber neuroplastik aut dem wege der transplantation. Arch Klin Chir. 1880;25:606.
-
- Maquet V. Martin D. Malgrange B. Franzen R. Schoenen J. Moonen G., et al. Peripheral nerve regeneration using bioresorbable macroporous polylactide scaffolds. J Biomed Mater Res. 2000;52:639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

