A secure distributed logistic regression protocol for the detection of rare adverse drug events
- PMID: 22871397
- PMCID: PMC3628043
- DOI: 10.1136/amiajnl-2011-000735
A secure distributed logistic regression protocol for the detection of rare adverse drug events
Abstract
Background: There is limited capacity to assess the comparative risks of medications after they enter the market. For rare adverse events, the pooling of data from multiple sources is necessary to have the power and sufficient population heterogeneity to detect differences in safety and effectiveness in genetic, ethnic and clinically defined subpopulations. However, combining datasets from different data custodians or jurisdictions to perform an analysis on the pooled data creates significant privacy concerns that would need to be addressed. Existing protocols for addressing these concerns can result in reduced analysis accuracy and can allow sensitive information to leak.
Objective: To develop a secure distributed multi-party computation protocol for logistic regression that provides strong privacy guarantees.
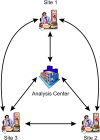
Methods: We developed a secure distributed logistic regression protocol using a single analysis center with multiple sites providing data. A theoretical security analysis demonstrates that the protocol is robust to plausible collusion attacks and does not allow the parties to gain new information from the data that are exchanged among them. The computational performance and accuracy of the protocol were evaluated on simulated datasets.
Results: The computational performance scales linearly as the dataset sizes increase. The addition of sites results in an exponential growth in computation time. However, for up to five sites, the time is still short and would not affect practical applications. The model parameters are the same as the results on pooled raw data analyzed in SAS, demonstrating high model accuracy.
Conclusion: The proposed protocol and prototype system would allow the development of logistic regression models in a secure manner without requiring the sharing of personal health information. This can alleviate one of the key barriers to the establishment of large-scale post-marketing surveillance programs. We extended the secure protocol to account for correlations among patients within sites through generalized estimating equations, and to accommodate other link functions by extending it to generalized linear models.
Conflict of interest statement
Figures

References
-
- Hoffman J, Doloresco F, Vermeulen L, et al. Projecting future drug expenditures. Am J Health Syst Pharm 2010;67:919–28 - PubMed
-
- Couzin J. Gaps in the safety Net. Science 2005;307:196–8 - PubMed
-
- Gough S. Post-marketing surveillance: a UK/European perspective. Curr Med Res Opin 2005;21:565–70 - PubMed
-
- Budnitz D, Pollock D, Weidenbach K, et al. National surveillance of emergency department visits for outpatient adverse drug events. JAMA 2006;296: 1858–66 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

