Critical pathogenic events underlying progression of neurodegeneration in glaucoma
- PMID: 22871543
- PMCID: PMC3472111
- DOI: 10.1016/j.preteyeres.2012.07.001
Critical pathogenic events underlying progression of neurodegeneration in glaucoma
Abstract
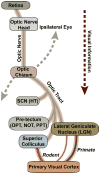
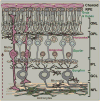
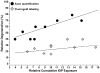
Glaucoma is a common optic neuropathy with a complex etiology often linked to sensitivity to intraocular pressure. Though the precise mechanisms that mediate or transduce this sensitivity are not clear, the axon of the retinal ganglion cell appears to be vulnerable to disease-relevant stressors early in progression. One reason may be because the axon is generally thin for both its unmyelinated and myelinated segment and much longer than the thicker unmyelinated axons of other excitatory retinal neurons. This difference may predispose the axon to metabolic and oxidative injury, especially at distal sites where pre-synaptic terminals form connections in the brain. This idea is consistent with observations of early loss of anterograde transport at central targets and other signs of distal axonopathy that accompany physiological indicators of progression. Outright degeneration of the optic projection ensues after a critical period and, at least in animal models, is highly sensitive to cumulative exposure to elevated pressure in the eye. Stress emanating from the optic nerve head can induce not only distal axonopathy with aspects of dying back neuropathy, but also Wallerian degeneration of the optic nerve and tract and a proximal program involving synaptic and dendritic pruning in the retina. Balance between progressive and acute mechanisms likely varies with the level of stress placed on the unmyelinated axon as it traverses the nerve head, with more acute insult pushing the system toward quicker disassembly. A constellation of signaling factors likely contribute to the transduction of stress to the axon, so that degenerative events along the length of the optic projection progress in retinotopic fashion. This pattern leads to well-defined sectors of functional depletion, even at distal-most sites in the pathway. While ganglion cell somatic drop-out is later in progression, some evidence suggests that synaptic and dendritic pruning in the retina may be a more dynamic process. Structural persistence both in the retina and in central projection sites offers the possibility that intrinsic self-repair pathways counter pathogenic mechanisms to delay as long as possible outright loss of tissue.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

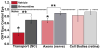


References
-
- Allan VJ. Cytoplasmic dynein. Biochem Soc Trans. 2011;39:1169–1178. - PubMed
-
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181. - PubMed
-
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. - PubMed
-
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. - PubMed
-
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

