Determination of severity of murine IgA nephropathy by glomerular complement activation by aberrantly glycosylated IgA and immune complexes
- PMID: 22871574
- PMCID: PMC3463632
- DOI: 10.1016/j.ajpath.2012.06.038
Determination of severity of murine IgA nephropathy by glomerular complement activation by aberrantly glycosylated IgA and immune complexes
Abstract
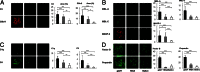
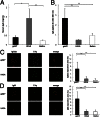
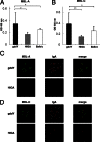
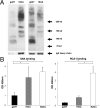
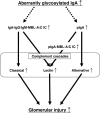
The pathogenic roles of glomerular deposition of components of the complement cascade in IgA nephropathy (IgAN) are not completely clarified. To investigate the pathologic role of complement pathways in IgAN, two IgAN-prone mouse models were examined. Grouped ddY (gddY) mice showed significant high proteinuria, severe glomerular lesions, and extracellular matrix expansion compared with high serum IgA (HIGA) mice but with similar intensity of glomerular IgA deposition. Glomerular activation of the classical, lectin, and alternative pathways was demonstrated by significantly stronger staining for complement (C)3, C5b-9, C1q, C4, mannose-binding lectin (MBL)-A/C, MBL-associated serine protease-2, and factor B and properdin in gddY mice than in HIGA mice. Similarly, the serum levels of IgA-IgG2a/IgM and IgA-MBL-A/C immune complexes and polymeric IgA were significantly higher in gddY mice than in HIGA mice. Moreover, the serum levels of aberrantly glycosylated IgA characterized by the binding of Sambucus nigra bark lectin and Ricinus communis agglutinin I were significantly higher in gddY mice than in HIGA mice. This aberrancy in glycosylation was confirmed by monosaccharide compositional analysis of purified IgA using gas-liquid chromatography. This study is the first to demonstrate that aberrantly glycosylated IgA may influence the formation of macromolecular IgA including IgA-IgG immune complexes and subsequent complement activation, leading to full progression of IgAN.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Glomerular deposition of mannose-binding lectin (MBL) indicates a novel mechanism of complement activation in IgA nephropathy.Nephrol Dial Transplant. 1998 Aug;13(8):1984-90. doi: 10.1093/ndt/13.8.1984. Nephrol Dial Transplant. 1998. PMID: 9719152
-
Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease.J Am Soc Nephrol. 2006 Jun;17(6):1724-34. doi: 10.1681/ASN.2005090923. Epub 2006 May 10. J Am Soc Nephrol. 2006. PMID: 16687629
-
Crucial Role of AIM/CD5L in the Development of Glomerular Inflammation in IgA Nephropathy.J Am Soc Nephrol. 2020 Sep;31(9):2013-2024. doi: 10.1681/ASN.2019100987. Epub 2020 Jul 1. J Am Soc Nephrol. 2020. PMID: 32611589 Free PMC article.
-
IgA glycosylation and immune complex formation in IgAN.Semin Immunopathol. 2021 Oct;43(5):669-678. doi: 10.1007/s00281-021-00883-8. Epub 2021 Sep 27. Semin Immunopathol. 2021. PMID: 34570260 Review.
-
Murine Models of Human IgA Nephropathy.Semin Nephrol. 2018 Sep;38(5):513-520. doi: 10.1016/j.semnephrol.2018.05.021. Semin Nephrol. 2018. PMID: 30177023 Review.
Cited by
-
Underweight Is an Independent Risk Factor for Renal Function Deterioration in Patients with IgA Nephropathy.PLoS One. 2016 Sep 9;11(9):e0162044. doi: 10.1371/journal.pone.0162044. eCollection 2016. PLoS One. 2016. PMID: 27611091 Free PMC article.
-
The kinetics of glomerular deposition of nephritogenic IgA.PLoS One. 2014 Nov 19;9(11):e113005. doi: 10.1371/journal.pone.0113005. eCollection 2014. PLoS One. 2014. PMID: 25409466 Free PMC article.
-
Diagnosis and activity assessment of immunoglobulin A nephropathy: current perspectives on noninvasive testing with aberrantly glycosylated immunoglobulin A-related biomarkers.Int J Nephrol Renovasc Dis. 2014 Oct 30;7:409-14. doi: 10.2147/IJNRD.S50513. eCollection 2014. Int J Nephrol Renovasc Dis. 2014. PMID: 25378944 Free PMC article. Review.
-
Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy.J Autoimmun. 2021 Mar;118:102593. doi: 10.1016/j.jaut.2021.102593. Epub 2021 Jan 25. J Autoimmun. 2021. PMID: 33508637 Free PMC article.
-
Enhanced auto-antibody production and Mott cell formation in FcμR-deficient autoimmune mice.Int Immunol. 2014 Dec;26(12):659-72. doi: 10.1093/intimm/dxu070. Epub 2014 Jul 3. Int Immunol. 2014. PMID: 24994818 Free PMC article.
References
-
- Berger J., Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol. 1968;74:694–695. - PubMed
-
- Allen A.C., Bailey E.M., Brenchley P.E., Buck K.S., Barratt J., Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60:969–973. - PubMed
-
- Hiki Y., Odani H., Takahashi M., Yasuda Y., Nishimoto A., Iwase H., Shinzato T., Kobayashi Y., Maeda K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. - PubMed
-
- Novak J., Julian B.A., Tomana M., Mestecky J. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2000;21:310–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

