Lack of response to unaligned chromosomes in mammalian female gametes
- PMID: 22871737
- PMCID: PMC3442912
- DOI: 10.4161/cc.21398
Lack of response to unaligned chromosomes in mammalian female gametes
Abstract
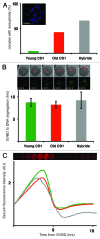
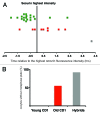
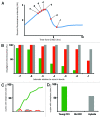
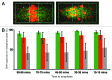
Chromosome segregation errors are highly frequent in mammalian female meiosis, and their incidence gradually increases with maternal age. The fate of aneuploid eggs is obviously dependent on the stringency of mechanisms for detecting unattached or repairing incorrectly attached kinetochores. In case of their failure, the newly formed embryo will inherit the impaired set of chromosomes, which will have severe consequences for its further development. Whether spindle assembly checkpoint (SAC) in oocytes is capable of arresting cell cycle progression in response to unaligned kinetochores was discussed for a long time. It is known that abolishing SAC increases frequency of chromosome segregation errors and causes precocious entry into anaphase; SAC, therefore, seems to be essential for normal chromosome segregation in meiosis I. However, it was also reported that for anaphase-promoting complex (APC) activation, which is a prerequisite for entering anaphase; alignment of only a critical mass of kinetochores on equatorial plane is sufficient. This indicates that the function of SAC and of cooperating chromosome attachment correction mechanisms in oocytes is different from somatic cells. To analyze this phenomenon, we used live cell confocal microscopy to monitor chromosome movements, spindle formation, APC activation and polar body extrusion (PBE) simultaneously in individual oocytes at various time points during first meiotic division. Our results, using oocytes from aged animals and interspecific crosses, demonstrate that multiple unaligned kinetochores and severe congression defects are tolerated at the metaphase to anaphase transition, although such cells retain sensitivity to nocodazole. This indicates that checkpoint mechanisms, operating in oocytes at this point, are essential for accurate timing of APC activation in meiosis I, but they are insufficient in detection or correction of unaligned chromosomes, preparing thus conditions for propagation of the aneuploidy to the embryo.
Figures

Comment in
-
New evidence that SAC can tolerate misaligned chromosomes in mouse oocytes.Cell Cycle. 2012 Sep 15;11(18):3356-7. doi: 10.4161/cc.21851. Epub 2012 Aug 23. Cell Cycle. 2012. PMID: 22918250 Free PMC article.
Similar articles
-
Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes.Development. 2012 Jun;139(11):1941-6. doi: 10.1242/dev.078352. Epub 2012 Apr 18. Development. 2012. PMID: 22513372 Free PMC article.
-
A single bivalent efficiently inhibits cyclin B1 degradation and polar body extrusion in mouse oocytes indicating robust SAC during female meiosis I.PLoS One. 2011;6(11):e27143. doi: 10.1371/journal.pone.0027143. Epub 2011 Nov 18. PLoS One. 2011. PMID: 22125605 Free PMC article.
-
Evaluation of the Spindle Assembly Checkpoint Integrity in Mouse Oocytes.J Vis Exp. 2022 Sep 13;(187):10.3791/64459. doi: 10.3791/64459. J Vis Exp. 2022. PMID: 36190266 Free PMC article.
-
Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error.Mutat Res. 2008 Mar 12;651(1-2):14-29. doi: 10.1016/j.mrgentox.2007.10.015. Epub 2007 Nov 9. Mutat Res. 2008. PMID: 18096427 Review.
-
New insights into the genetic regulation of homologue disjunction in mammalian oocytes.Cytogenet Genome Res. 2011;133(2-4):209-22. doi: 10.1159/000324118. Epub 2011 Feb 17. Cytogenet Genome Res. 2011. PMID: 21335952 Free PMC article. Review.
Cited by
-
Age-related differences in the translational landscape of mammalian oocytes.Aging Cell. 2020 Oct;19(10):e13231. doi: 10.1111/acel.13231. Epub 2020 Sep 20. Aging Cell. 2020. PMID: 32951297 Free PMC article.
-
Spc24 is required for meiotic kinetochore-microtubule attachment and production of euploid eggs.Oncotarget. 2016 Nov 1;7(44):71987-71997. doi: 10.18632/oncotarget.12453. Oncotarget. 2016. PMID: 27713128 Free PMC article.
-
Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes.Development. 2014 Jan;141(1):199-208. doi: 10.1242/dev.100206. Development. 2014. PMID: 24346700 Free PMC article.
-
Reduced ability to recover from spindle disruption and loss of kinetochore spindle assembly checkpoint proteins in oocytes from aged mice.Cell Cycle. 2014;13(12):1938-47. doi: 10.4161/cc.28897. Epub 2014 Apr 23. Cell Cycle. 2014. PMID: 24758999 Free PMC article.
-
The spindle checkpoint and chromosome segregation in meiosis.FEBS J. 2015 Jul;282(13):2471-87. doi: 10.1111/febs.13166. Epub 2015 Jan 12. FEBS J. 2015. PMID: 25470754 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
