Cysteinyl leukotriene 1 receptor expression associated with bronchial inflammation in severe exacerbations of COPD
- PMID: 22871757
- PMCID: PMC3425162
- DOI: 10.1378/chest.11-1581
Cysteinyl leukotriene 1 receptor expression associated with bronchial inflammation in severe exacerbations of COPD
Abstract
Background: Cysteinyl leukotriene 1 (CysLT1) receptor expression is known to be increased in the airway mucosa of patients with asthma, especially during exacerbations; however, nothing is known of its expression in COPD.
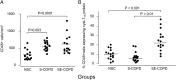
Methods: We applied immunohistochemistry and in situ hybridization to endobronchial biopsies to determine inflammatory cell CysLT1 receptor protein and mRNA expression in the following: (1) 15 nonsmoker control subjects (NSC), (2) 16 smokers with moderate to severe COPD in its stable phase (S-COPD), and (3) 15 smokers with COPD hospitalized for a severe exacerbation (SE-COPD).
Results: The total number of bronchial mucosal inflammatory cells (CD45+) and those expressing CysLT1 receptor protein were significantly greater in SE-COPD (CysLT1 receptor protein: median [range] = 139 [31-634]) as compared with S-COPD (32 [6-114]) or NSC (16 [4-66]) (P < .001 for both). CysLT1 receptor gene expression showed similar differences. A greater proportion of CD451 cells expressed CysLT1 receptor protein in SE-COPD (median [range] = 22% [8-81]) compared with S-COPD (10% [4-32]) (P < .03) or NSC (7% [1-19]) (P < .002). In SE-COPD, the relative frequencies of CysLT1 receptor-expressing cells were as follows: tryptase1 mast cells > CD681 monocytes/macrophage > neutrophils > CD201 B lymphocytes = EG21 eosinophils. Moreover, there were positive correlations between the numbers of cells expressing CysLT1 receptor protein and the numbers of CD451 cells (r = 0.78; P < .003) and tryptase1 mast cells (r = 0.62; P < .02).
Conclusions: Bronchial mucosal CysLT1 receptor-positive inflammatory cells are present in the bronchial mucosa in COPD in greatest number in those experiencing a severe exacerbation.
Figures







Similar articles
-
Localization and upregulation of cysteinyl leukotriene-1 receptor in asthmatic bronchial mucosa.Am J Respir Cell Mol Biol. 2005 Dec;33(6):531-40. doi: 10.1165/rcmb.2005-0124OC. Epub 2005 Aug 25. Am J Respir Cell Mol Biol. 2005. PMID: 16123393
-
Does leptin play a cytokine-like role within the airways of COPD patients?Eur Respir J. 2005 Sep;26(3):398-405. doi: 10.1183/09031936.05.00092404. Eur Respir J. 2005. PMID: 16135719
-
Bronchial mucosal dendritic cells in smokers and ex-smokers with COPD: an electron microscopic study.Thorax. 2008 Feb;63(2):108-14. doi: 10.1136/thx.2007.078253. Epub 2007 Sep 17. Thorax. 2008. PMID: 17875567
-
Functional characterisation of receptors for cysteinyl leukotrienes in smooth muscle.Acta Physiol Scand Suppl. 1998 Mar;641:1-55. Acta Physiol Scand Suppl. 1998. PMID: 9597121 Review.
-
Cysteinyl leukotriene receptors.Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:587-97. doi: 10.1016/s0090-6980(02)00057-6. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432945 Review.
Cited by
-
Pulmonary epithelial cancer cells and their exosomes metabolize myeloid cell-derived leukotriene C4 to leukotriene D4.J Lipid Res. 2016 Sep;57(9):1659-69. doi: 10.1194/jlr.M066910. Epub 2016 Jul 19. J Lipid Res. 2016. PMID: 27436590 Free PMC article.
-
Influence of the Lung Microbiota Dysbiosis in Chronic Obstructive Pulmonary Disease Exacerbations: The Controversial Use of Corticosteroid and Antibiotic Treatments and the Role of Eosinophils as a Disease Marker.J Clin Med Res. 2019 Oct;11(10):667-675. doi: 10.14740/jocmr3875. Epub 2019 Oct 4. J Clin Med Res. 2019. PMID: 31636780 Free PMC article. Review.
-
The Effectiveness of Anti-leukotriene Agents in Patients with COPD: A Systemic Review and Meta-analysis.Lung. 2015 Aug;193(4):477-86. doi: 10.1007/s00408-015-9743-5. Epub 2015 May 14. Lung. 2015. PMID: 25972156
-
High blood eosinophils predict the risk of COPD exacerbation: A systematic review and meta-analysis.PLoS One. 2024 Oct 3;19(10):e0302318. doi: 10.1371/journal.pone.0302318. eCollection 2024. PLoS One. 2024. PMID: 39361621 Free PMC article.
-
Baicalin Ameliorates Radiation-Induced Lung Injury by Inhibiting the CysLTs/CysLT1 Signaling Pathway.Evid Based Complement Alternat Med. 2022 Jun 24;2022:2765354. doi: 10.1155/2022/2765354. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35783527 Free PMC article.
References
-
- Holgate ST, Peters-Golden M. Introduction: the anti-inflammatory role of cysteinyl leukotriene receptor antagonists in asthma. J Allergy Clin Immunol. 2003;111(suppl 1):S1-S4 - PubMed
-
- Krawiec ME, Wenzel SE. Leukotriene inhibitors and non-steroidal therapies in the treatment of asthma. Expert Opin Pharmacother. 2001;2(1):47-65 - PubMed
-
- Drazen JM. Anti-leukotrienes as novel anti-inflammatory treatments in asthma. Adv Exp Med Biol. 2002;507:217-221 - PubMed
-
- Parameswaran K, Liang H, Fanat A, Watson R, Snider DP, O’Byrne PM. Role for cysteinyl leukotrienes in allergen-induced change in circulating dendritic cell number in asthma. J Allergy Clin Immunol. 2004;114(1):73-79 - PubMed
-
- Jeffery PK. The roles of leukotrienes and the effects of leukotriene receptor antagonists in the inflammatory response and remodelling of allergic asthma. Clin Exp Allergy Rev. 2001;1(2):148-153
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

