Geographical variations in current clinical practice on transfusions and iron chelation therapy across various transfusion-dependent anaemias
- PMID: 22871821
- PMCID: PMC3557481
- DOI: 10.2450/2012.0012-12
Geographical variations in current clinical practice on transfusions and iron chelation therapy across various transfusion-dependent anaemias
Abstract
Background and objectives: Many patients with chronic anaemia require blood transfusions as part of their treatment regimen. As a result, iron overload will inevitably develop if not adequately managed by iron chelation therapy. There are many guidelines relating to transfusion and chelation practices for patients with transfusion-dependent anaemia; however, there is a lack of information on how treatment practices differ around the world. The objective of this manuscript is to highlight key features of current transfusion and chelation management, including similarities and differences across various anaemias and between geographical regions worldwide.
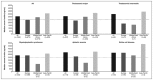
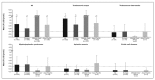
Materials and methods: Data collected at study entry to the multicentre Evaluation of Patients' Iron Chelation with Exjade (EPIC) study, which recruited 1,744 patients with a variety of transfusion-dependent anaemias across 23 countries from three geographic regions, were assessed. These analyses compared transfusion and chelation treatment prior to the start of study treatment, together with iron burden assessed at study entry by serum ferritin, liver iron concentration and labile plasma iron levels.
Results and conclusions: Data show that transfusion and iron chelation practices differ between anaemias and between geographical regions; this may be linked to availability and accessibility of transfusion and chelation therapy, patients' compliance, physicians' attitudes, costs and use of treatment guidelines. Approximately 60% of these transfusion-dependent patients were severely iron overloaded with a serum ferritin level over 2,500 ng/mL, indicating that the risks of iron burden may have been underestimated and current iron chelation therapy, if considered, may not have been adequate to control iron burden.
Figures






References
-
- Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. - PubMed
-
- Adams RJ, Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–78. - PubMed
-
- Hellström-Lindberg E. Management of anemia associated with myelodysplastic syndrome. Semin Hematol. 2005;42:S10–S13. - PubMed
-
- Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. - PubMed
-
- Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: a report from the multi-center study of iron overload. Am J Hematol. 2007;82:255–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
