Preclinical evaluation of reconsolidation blockade by clonidine as a potential novel treatment for posttraumatic stress disorder
- PMID: 22871915
- PMCID: PMC3499710
- DOI: 10.1038/npp.2012.145
Preclinical evaluation of reconsolidation blockade by clonidine as a potential novel treatment for posttraumatic stress disorder
Abstract
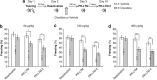
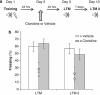
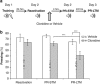
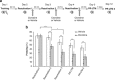
Exposure to traumatic events can lead to posttraumatic stress disorder (PTSD). Current PTSD treatments typically only produce partial improvement. Hence, there is a need for preclinical research to identify new candidate drugs and to develop novel therapeutic approaches. Animal studies have indicated that fear memories can be weakened by blocking restabilization after retrieval, a process known as reconsolidation. Furthermore, evidence suggests that there are important alterations of the noradrenergic system in PTSD, and hence it may be of interest to study drugs that target this pathway. Here, we investigated the efficacy of clonidine, an α₂-adrenoreceptor agonist, to block reconsolidation in an animal model of persistent traumatic memories. Using an auditory fear conditioning paradigm in rats, we tested the efficacy of clonidine to weaken fear memory retention when administered systemically after retrieval. We evaluated dosage, number of treatments, and specificity in reconsolidation blockade. We found that postretrieval administration of clonidine disrupts fear-related memories in a dose-dependent manner and that two treatments are sufficient for maximal memory impairment. Furthermore, we determined that this effect is long lasting and specific to reconsolidation processes as shown by the selectivity to affect reactivated memories and the absence of spontaneous recovery and of postreactivation short-term memory impairment. Our results demonstrate the efficacy of systemic administration of clonidine following retrieval to persistently disrupt fear memory retention through reconsolidation blockade. This study provides important preclinical parameters for future therapeutic strategies involving clonidine to block reconsolidation as a novel treatment for PTSD symptoms.
Figures

References
-
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–1239. - PubMed
-
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. - PubMed
-
- Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13:72–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

