Effect of the VKORC1 D36Y variant on warfarin dose requirement and pharmacogenetic dose prediction
- PMID: 22871975
- PMCID: PMC3461592
- DOI: 10.1160/TH12-03-0151
Effect of the VKORC1 D36Y variant on warfarin dose requirement and pharmacogenetic dose prediction
Abstract
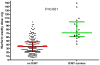
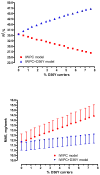
Pharmacogenetic dosing algorithms help predict warfarin maintenance doses, but their predictive performance differs in different populations, possibly due to unsuspected population-specific genetic variants. The objectives of this study were to quantify the effect of the VKORC1 D36Y variant (a marker of warfarin resistance previously described in 4% of Ashkenazi Jews) on warfarin maintenance doses and to examine how this variant affects the performance of the International Warfarin Pharmacogenetic Consortium (IWPC) dose prediction model. In 210 Israeli patients on chronic warfarin therapy recruited at a tertiary care centre, we applied the IWPC model and then added D36Y genotype as covariate to the model (IWPC+D36Y) and compared predicted with actual doses. Median weekly warfarin dose was 35 mg (interquartile range [IQR], 24.5 to 52.5 mg). Among 16 heterozygous D36Y carriers (minor allele frequency = 3.8%), warfarin weekly dose was increased by a median of 43.7 mg (IQR, 40.5 to 47.2 mg) compared to non-carriers after adjustment for all IWPC parameters, a greater than two-fold dose increase. The IWPC model performed suboptimally (coefficient of determination R²=27.0%; mean absolute error (MAE), 14.4 ± 16.2 mg/week). Accounting for D36Y genotype using the IWPC+D36Y model resulted in a significantly better model performance (R²=47.2%, MAE=12.6 ± 12.4 mg/week). In conclusion, even at low frequencies, variants with a strong impact on warfarin dose may greatly decrease the performance of a commonly used dose prediction model. Unexpected discrepancies of the performance of universal prediction models in subpopulations should prompt searching for unsuspected confounders, including rare genetic variants.
Conflict of interest statement
Dr. Gak holds a 40% stake in patent PCT no. IL2007/000405, filed in March 2007, entitled “Methods and kits for determining predisposition to warfarin resistance”, which includes the
Figures

Similar articles
-
Validation of pharmacogenetic algorithms and warfarin dosing table in Egyptian patients.Int J Clin Pharm. 2012 Dec;34(6):837-44. doi: 10.1007/s11096-012-9678-3. Epub 2012 Jul 27. Int J Clin Pharm. 2012. PMID: 22851439
-
Accuracy assessment of pharmacogenetically predictive warfarin dosing algorithms in patients of an academic medical center anticoagulation clinic.J Thromb Thrombolysis. 2010 Aug;30(2):220-5. doi: 10.1007/s11239-010-0459-3. J Thromb Thrombolysis. 2010. PMID: 20204461
-
Clinical relevance of VKORC1 (G-1639A and C1173T) and CYP2C9*3 among patients on warfarin.J Clin Pharm Ther. 2012 Apr;37(2):232-6. doi: 10.1111/j.1365-2710.2011.01262.x. Epub 2011 Apr 20. J Clin Pharm Ther. 2012. PMID: 21507031
-
Pharmacogenetics of oral anticoagulants: a basis for dose individualization.Clin Pharmacokinet. 2008;47(9):565-94. doi: 10.2165/00003088-200847090-00002. Clin Pharmacokinet. 2008. PMID: 18698879 Review.
-
Role of warfarin pharmacogenetic testing in clinical practice.Pharmacogenomics. 2010 Mar;11(3):439-48. doi: 10.2217/pgs.10.8. Pharmacogenomics. 2010. PMID: 20402581 Review.
Cited by
-
The Prevalence of Pharmacogenomics Variants and Their Clinical Relevance Among the Pakistani Population.Evol Bioinform Online. 2022 Apr 24;18:11769343221095834. doi: 10.1177/11769343221095834. eCollection 2022. Evol Bioinform Online. 2022. PMID: 35497687 Free PMC article.
-
Frequency of Common VKORC1 Polymorphisms and Their Impact on Warfarin Dose Requirement in Pakistani Population.Clin Appl Thromb Hemost. 2018 Mar;24(2):323-329. doi: 10.1177/1076029616680478. Epub 2016 Nov 22. Clin Appl Thromb Hemost. 2018. PMID: 27879469 Free PMC article.
-
One Rare Warfarin Resistance Case and Possible Mechanism Exploration.Pharmgenomics Pers Med. 2023 Jun 20;16:609-615. doi: 10.2147/PGPM.S404474. eCollection 2023. Pharmgenomics Pers Med. 2023. PMID: 37359384 Free PMC article.
-
VKORC1 Asp36Tyr geographic distribution and its impact on warfarin dose requirements in Egyptians.Thromb Haemost. 2013 Jun;109(6):1045-50. doi: 10.1160/TH12-10-0789. Epub 2013 Mar 21. Thromb Haemost. 2013. PMID: 23571513 Free PMC article.
-
Pharmacogenetics in Jewish populations.Drug Metabol Drug Interact. 2014;29(4):221-33. doi: 10.1515/dmdi-2013-0069. Drug Metabol Drug Interact. 2014. PMID: 24867283 Free PMC article. Review.
References
-
- Kurnik D, Loebstein R, Halkin H, et al. 10 years of oral anticoagulant pharmacogenomics: what difference will it make? A critical appraisal. Pharmacogenomics. 2009;10:1955–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

